
The optimal neoadjuvant treatment of locally advanced esophageal cancer
Introduction
Esophagectomy is still the cornerstone of intentionally curative treatment in patients with locally advanced esophageal cancer. Outcomes of esophageal cancer surgery have been reported since 1950. Between 1950 and 2000, 5-year overall survival (OS) of patients after surgery alone has improved from approximately 12% to 39% (1-4). Probably, this might be explained by better patient selection, improvement in perioperative care and introduction of more radical resections (e.g., transthoracic resection with extended en bloc lymphadenectomy). However, the proportion of patients with microscopically positive resection margins; including circumferential resection margin (R1) was seen in 25–30% of the patients (3,5). Furthermore, after primary esophagectomy, nearly half of the patients developed distant metastases and nearly 40% of patients developed locoregional recurrences (6,7). In order to decrease locoregional- and distant recurrences and irradical resections, several neoadjuvant therapies have been tested.
Neoadjuvant therapies in esophageal cancer mostly consist of chemotherapy-, chemoradiotherapy and more recently monoclonal antibodies (mAbs). Both chemotherapy and chemoradiotherapy followed by esophagectomy improve OS compared to esophagectomy alone (6,8,9). In large parts of the western world neoadjuvant chemoradiotherapy followed by esophagectomy has been adopted as standard intentionally curative treatment for esophageal cancer. However, some countries advocate the use of chemotherapy as standard therapy prior to surgery. Currently, controversy exist on which therapy is superior. Radiotherapy mostly relies on locoregional disease control while chemotherapy has the potential to also eliminate micrometastases and thus, possibly prevent outgrowth of metastases in other organs. This review aims at providing an overview of the currently available neoadjuvant therapies and as such, to determine the optimal neoadjuvant treatment for locally advanced esophageal cancer.
Chemotherapy
Chemotherapy acts both locally and systemically by downstaging of the primary tumor to increase the chance of a radical resection and elimination of (subclinical) micrometastases to decrease the risk of development of distant metastases. Chemotherapy is divided in several subclasses according to the mechanisms of action. For (gastro)esophageal cancer, mostly the platinum-based chemotherapeutics, taxanes and pyrimidine analogues are used. Platinum-based chemotherapeutics (e.g., cisplatin, oxaliplatin and carboplatin) induce DNA damage by production of inter- and intrastrand DNA crosslinks which inhibits the synthesis of DNA, RNA and proteins (10). As a result, platinum-based chemotherapeutics tend to eliminate the proliferating (carcinogenic) cells. Taxanes (e.g., paclitaxel and docetaxel) are a class of chemotherapeutics synthetically constructed from derivatives of the needles of Yew plants (11). Depolymerization of the cytoskeletal structures in a cell is essential for cell proliferation. Taxanes stabilize the cytoskeletal structures and thus, prevent depolymerization and cell division, resulting in cell-cycle arrest. Docetaxel is more potent than paclitaxel in enhancing the stability of cytoskeletal structures and is also able to induce apoptosis. The pyrimidine analogues (e.g., 5-fluorouracil) are competing structural analogs to naturally occurring metabolites that are involved in the synthesis of DNA and RNA (12). They are most effective against cells that are in the DNA duplication phase of the cell-cycle. Consequently, these cytostatic agents tend to eliminate cells with a high growth fraction. The addition of chemotherapy to the treatment-regimen of patients with gastric-, junctional- and esophageal cancer is mainly based on two large randomized clinical trials; the MAGIC-trial and the OEO2-trial (8,13,14).
The MAGIC-trial was published in 2006. Some 503 patients were randomized between 1994–2002 with resectable adenocarcinoma of the stomach, gastroesophageal junction or lower esophagus between perioperative chemotherapy followed by surgery and surgery alone (13). Both pre- and postoperatively, three cycles were administered consisting of epirubicin (60 mg/m2) and cisplatin (60 mg/m2) on day 1 and a continuous infusion of fluorouracil (200 mg/m2) for 21 days. Of the 237 patients that started with chemotherapy, 215 patients (90.7%) completed the preoperative cycles and 137 (57.8%) subsequently started the postoperative cycles. Eventually, 104 (43.9%) patients underwent all chemotherapy-cycles. Relatively high rates of grade 3–4 adverse events were seen, most frequently granulocytopenia (23.8% preoperatively and 27.8% postoperatively). No information was reported concerning pathologically complete response rate or radical resection rate. Median follow-up was 47 and 49 months for the chemotherapy plus surgery and surgery only group, respectively. After addition of perioperative chemotherapy, three and 5-year OS significantly improved from 31% to 44% and 23% to 36.3% respectively. However, since only a minority of patients had esophageal- (14.5%) or junctional (11%) cancer, the results of this study cannot indisputably be extrapolated to patients with esophageal cancer.
The largest trial including mostly esophageal cancer patients undergoing neoadjuvant chemotherapy followed by surgery versus surgery alone was the British OEO2-trial (8,14). This trial randomized 802 patients in the period 1992 and 1998 between two 4-day cycles of cisplatin (80 mg/m2), 3 weeks apart, and continuous infusion of fluorouracil (1,000 mg/m2) for 4 days followed by surgery versus surgery alone. Nearly one-third of the patients had squamous cell carcinoma and two-thirds had adenocarcinoma. Of 372 patients that started pretreatment, 350 (94%) underwent both cycles. Only 65% of patients undergoing neoadjuvant chemotherapy and surgery had an R0 resection and no tumor could be detected in the resected esophagus in 4%, suggesting a pathologically complete response (pCR, i.e., no vital tumor cells in the resection specimen). The median follow-up was approximately 37.4 months. After the addition of neoadjuvant chemotherapy, 3- and 5-year OS significantly improved from 25% to 32% and from 13% to 23%, respectively. The benefit in OS after addition of chemotherapy was confirmed in the publication of the long-term results of this study. Surprisingly, there was no difference in rate of distant metastases between the two groups, suggesting a modest systemic effect of this chemotherapy regimen.
However, the results of the OEO2-trial were not confirmed by the RTOG-trial 8911 that was performed in the USA (15,16). Approximately in the same period of time the study randomized 440 patients with esophageal cancer between three cycles of cisplatin (100 mg/m2) on day 1 and continuous infusion of fluorouracil (1,000 mg/m2) for 4 days followed by surgery versus surgery alone. Approximately half of the patients had squamous cell carcinoma and half of the patients had adenocarcinoma. Of all patients that underwent chemotherapy followed by surgery, 78% had R0-resection and 2.5% of patients that underwent at least one cycle of chemotherapy achieved pCR. In contrast to the OEO2-trial, OS did not improve after addition of chemotherapy prior to surgery. The median follow-up was 46.5 months. Patients undergoing preoperative chemotherapy followed by surgery or surgery alone had a 3-year OS of 23% versus 26%, respectively and a 5-year OS of 22% versus 19%, respectively. In the RTOG-trial 8911, 133 of the 233 patients (57%) that were assigned to the preoperative chemotherapy group underwent surgery compared to 361 of the 400 patients (90%) in the OEO2-trial. This could be due to the high toxicity that was seen in the RTOG-trial 8911; ≥ grade 3 neutropenia in 29% of patients. No results were reported concerning graded adverse events in the OEO2-trial. However, the authors reported that in 8% of the patients that underwent neoadjuvant chemotherapy, the total dose of chemotherapy was reduced due to neutropenia. This suggests that the chemotherapy regimen in the RTOG-trial was more toxic than the chemotherapy regimen used in the OEO2-trial. This could be a possible explanation for the differences between the two studies.
Recently, the preliminary results of the FLOT4-trial, which were presented at the American Society of Clinical Oncology meeting in 2017, have drawn great attention (17). This multicenter phase III study included 716 patients with adenocarcinoma of the stomach or gastroesophageal junction. One group of patients was treated with 3 preoperative and 3 postoperative cycles of epirubicin (50 mg/m2) and cisplatin (60 mg/m2) on day 1 and either fluorouracil (200 mg/m2) as continuous infusion or capecitabine (1,250 mg/m2) on days 1 to 21 orally (ECF/ECX) according to the MAGIC regimen (13). The second group of patients were treated with 4 preoperative and 4 postoperative 2 week-cycles of fluorouracil (2,600 mg/m2), leucovorin (100 mg/m2), oxaliplatin (85 mg/m2) and docetaxel (50 mg/m2) (FLOT), all as continuous infusion. The preliminary results indicate that 91% of the patients undergoing ECF/ECX completed the preoperative cycles and 37% completed the postoperative cycles, versus 90% and 50% of patients undergoing the FLOT regimen. Most importantly, median OS significantly improved from 35 months for ECF/ECX to 50 months for FLOT after a median follow-up 43 months (HR 0.77; 95% CI, 0.63–0.94; P=0.012). Three-year OS rate was 48% for patients undergoing ECF/ECX versus 57% for patients undergoing FLOT. However, until all results concerning survival, toxicity profiles and methods are published, caution is needed to draw final conclusions.
The Japanese JCOG9204 trial showed that also adjuvant chemotherapy resulted in significantly improved disease-free survival in in patients with esophageal squamous cell cancer (18). This resulted in the initiation of the JCOG9907-trial that compared neoadjuvant chemotherapy versus adjuvant chemotherapy (18,19). The JCOG9907-trial randomized 330 patients with squamous cell cancer between either neoadjuvant or adjuvant chemotherapy treatment consisting of two cycles of cisplatin (80 mg/m2) intravenously on day 1, and 5-fluorouracil (800 mg/m2) as continuous infusion on days 1 to 5. Only patients with a node-positive status (pN1, according to the 6th edition of the TNM-staging) received adjuvant chemotherapy. In the neoadjuvant chemotherapy group, 95% of patients undergoing surgery had R0-resection versus 91% in the adjuvant chemotherapy group. Toxicity of the used regimen was mild with most common occurring grade 3 or 4 adverse events in 3% and 5% (leukopenia) of patients undergoing neoadjuvant and adjuvant chemotherapy, respectively. Complete responses were observed in 2.5% of patients that underwent surgery. The Data and Safety Monitoring Board recommended early publication of the results after OS showed to be superior in patients undergoing neoadjuvant chemotherapy (HR 0.64; 95% CI, 0.45–0.91; P=0.01) at an interim analysis. The results of the final analysis reported a significantly improved 5-year OS in patients undergoing neoadjuvant chemotherapy from 43% to 55%.
A chemotherapy regimen that has long been solely used and is developed in Japan is S-1, an oral fluoropyrimidine alternative for infusional 5-fluorouracil. This regimen, consisting of tegafur, gimeracil and oteracil potassium, is widely being used in Asian countries for the treatment of advanced gastric cancer, mostly based on several phase II studies (20-22). Furthermore, S-1 has been suggested to be effective in the treatment of advanced esophageal cancer patients in Japan (23,24). One of the substances of S-1, tegafur, is a prodrug which is converted to the active form 5-fluorouracil by the liver enzyme CYP2A6 (25). However, patients in Japan more frequently harbor variants of CYP2A6 which results in a lower concentration of the active form 5-fluorouracil in the plasma of the patient because of lower clearance of tegafur (26). When a phase I study was conducted in the United States, this resulted in dose-limiting toxicities (27). The phase III FLAGS-trial that was conducted in the United States compared S-1 (50 mg/m2) in two daily doses for 21 days and cisplatin (75 mg/m2) on day 1 for 28 days versus 5-fluorouracil (1,000 mg/m2) as continuous infusion for 120 hours with cisplatin (100 mg/m2) on day 1 for 28 days (28). Although no difference in OS was observed, administration of S-1/cisplatin resulted in a significantly improved safety profile compared to 5-fluorouracil/cisplatin. As such, S-1 was introduced as feasibly oral alternative for 5-fluorouracil for the treatment of advanced gastric- and gastroesophageal junctional cancer among Western countries. It is postulated that the difference in presence of variant CYP2A6 in patients in Japan and Western countries resulted in the differences in toxicity profiles and thus, the delay of application of S-1 in Western countries.
Chemoradiotherapy
Trimodality treatment, consisting of chemotherapy, radiotherapy and surgery was introduced mainly for the treatment of esophageal cancer after the RTOG-8501 study reported an advantage of chemoradiotherapy over radiotherapy alone (29,30). In addition to the systemic effects, chemotherapy has shown its efficacy in potentiating the anti-tumor effects of radiotherapy. For platinum analogues such as cisplatin and carboplatin, the enhanced elimination of tumor cells, if continued by radiotherapy is believed to depend on a variety of mechanisms including radiation-induced increase in cellular platinum uptake, inhibition of DNA-repair and enhanced cell-cycle arrest (31-33).
The first adequately powered randomized controlled trial that reported on the outcomes of neoadjuvant chemoradiotherapy (nCRT) followed by surgery versus surgery alone was published in 1996 by Walsh et al. (34). Between 1990 and 1995, 113 patients with adenocarcinoma of the esophagus were randomized between nCRT consisting of two cycles of fluorouracil (15 mg/kg) on days 1 to 5 and cisplatin (75 mg/m2) on day 7 concurrently with 40 Gy radiotherapy in 15 fractions followed by surgery versus surgery alone. The treatment-related morbidity was low (10% grade 3 and 3.3% grade 4 adverse events) in patients undergoing nCRT. Of 52 patients that underwent nCRT and surgery, 13 (25%) reached pCR. The median follow-up was 18 months. After addition of nCRT, three-year OS significantly improved from 6% to 32%. This was one of the first studies that provided robust evidence that nCRT followed by surgery provides a significant survival advantage over surgery alone in patients with adenocarcinoma.
The results of another significant trial that reported on the outcomes of nCRT treatment in esophageal cancer were published in 2012 (6,9). This Dutch CROSS-trial randomized 366 patients between nCRT that consisted of five weekly cycles of carboplatin (AUC 2 mg/mL) on day 1 and paclitaxel (50 mg/m2) on day 1 with concurrent 41.4 Gy in 23 fractions followed by surgery versus surgery alone. This nCRT-regimen was associated with modest presence of ≥ grade 3 adverse events with leukopenia as most frequently occurring adverse event in 6% of the patients undergoing nCRT. One patient died after nCRT due to bleeding from an esophago-aortic fistula, in the absence of thrombocytopenia. A modest effect on the health-related quality of life was reported (35,36). After nCRT, 92% of patients had R0-resection, compared to 69% in the surgery alone group. Overall, nearly one-third of the patients achieved pCR (23% in patients with adenocarcinoma and 49% in patients with squamous cell carcinoma). Most importantly, 5-year OS improved from 33% to 47% after addition of nCRT. Since the publication of the results of the CROSS-trial, nCRT has been part of standard treatment for locally advanced esophageal cancer in large parts of the western world. However, although the effects of nCRT on squamous cell carcinoma were larger, only a fraction of the patients in the CROSS-trial had squamous cell carcinoma (41 in the nCRT group and 43 in the surgery group) which makes it hard to widely extrapolate the results of the study for this subgroup. Very recently, Yang et al. published the NEOCRTEC5010-trial that randomized 451 patients between 2007 and 2014 with squamous cell carcinoma between nCRT consisting of two three-weekly cycles of vinorelbine (25 mg/m2) on days 1 to 8 and 75 mg/m2 cisplatin on day 1 or 25 mg/m2 on days 1 to 4, with concurrent 40 Gy radiotherapy in 20 fractions, followed by surgery versus surgery alone (37). A pathologically complete response was achieved in 43.2% of patients undergoing nCRT. The median follow-up was 41 months for patients undergoing nCRT followed by surgery and 34.6 months for patients undergoing surgery alone. After addition of nCRT, 3-year OS significantly improved from 60% to 69% and 5-year OS significantly improved from 51% to 61%. The NEOCRTEC5010-trial provides strong evidence in favor of nCRT in adequately sized groups of patients with squamous cell cancer. This study reported R1-resections in only 7.9% of patients undergoing surgery alone versus 31% in the CROSS-trial. However, the CROSS-trial defined R1 resection as having positive proximal, distal and/or circumferential resection margins. The NEOCRTEC5010-trial did not include positive circumferential margins as R1 resection. This could be an explanation for the discrepancy in rate of R1 between the two trials. Furthermore, in this study, a considerable number of patients undergoing nCRT developed grade 3 and/or 4 hematologic adverse events (54.3%). Another study using a similar nCRT-regimen also reported high rates of grade 3–4 adverse hematological events, mostly grade 4 neutropenia (23%), in the treatment of metastatic esophageal squamous cell cancer (38). In the NEOCRTEC5010-trial, two different cisplatin-regimens were used. Interestingly, administrating two cycles of cisplatin 25 mg/m2 on days 1 to 4 resulted in significantly higher grade 3–4 leukopenia and/or neutropenia than the alternative regimen of 75 mg/m2 on day 1. The authors state that 82.6% of patients completed the whole multimodality therapy. However, the supplementary material suggests that only 61.6% underwent the total dose of the 25 mg/m2 cisplatin-regimen. Even after exclusion of the more toxic cisplatin-regimen of 25 mg/m2, compliance to the chemotherapy-regimen seems relatively low, since only 56.5% of patients underwent the total dose of the vinorelbine-regimen. Hence, the reported 82.6% who completed multimodality therapy most probably includes patients in whom the total chemotherapy dose was reduced. This resulted in an overall compliance to the total dose chemotherapy of at most 56.5% (versus an overall compliance to the total dose chemotherapy of 91% in the CROSS-trial). Although no direct comparison has been made, these results suggest that the proposed regimen seems relatively toxic compared to the CROSS-regimen.
Another treatment strategy that has been investigated is induction chemotherapy followed by nCRT. A phase II trial randomized patients between induction chemotherapy followed by nCRT versus nCRT alone (39). Induction chemotherapy consisted of 4-week cycles of oxaliplatin (100 mg/m2) and fluorouracil (2,200 mg/m2) as continuous infusion for 48 hours, both on days 1 and 15. nCRT consisted of 5 weekly cycles of oxaliplatin (40 mg/m2) intravenously once a week with fluorouracil (250 mg/m2) as continuous infusion for days 1 to 5 concurrently with 50.4 Gy radiotherapy in 28 fractions. None of the grade 3–4 adverse events were reported in more than 5% of patients. The primary outcome of this study was the rate of pCR. Fourteen of 54 (26%) patients that underwent induction chemotherapy followed by nCRT and surgery had pCR versus 13% of patients that underwent nCRT followed by surgery (P=0.094). Moreover, no differences were seen in OS between patients undergoing nCRT followed by surgery with or without prior induction chemotherapy (P=0.69). However, a secondary analysis of this randomized trial reported that induction chemotherapy significantly prolonged OS in patients that had well to moderately differentiated tumors (40). Furthermore, having well or moderately differentiated tumors while undergoing induction chemotherapy prior to nCRT and surgery was an independent prognostic factor in multivariate analysis. Possibly, a three-step strategy consisting of induction chemotherapy, nCRT and surgery could be beneficial in a subset of patients with locally advanced esophageal cancer. However, prospective evaluation is needed.
Chemotherapy versus chemoradiotherapy
Two neoadjuvant treatments, chemotherapy and chemoradiotherapy, for both squamous cell- and adenocarcinoma have been adopted after the publication of the OEO2, MAGIC- and CROSS-trials. Some direct comparisons have been made between these neoadjuvant treatments but these studies were of moderate to poor quality.
Earlier meta-analyses were published on this topic and suggested that both chemotherapy and chemoradiotherapy are of benefit for adenocarcinoma of the esophagus while in squamous cell carcinoma, the advantage for chemoradiotherapy is greater than that of chemotherapy (41,42). A larger effect on all-cause mortality was observed for nCRT versus surgery alone (HR 0.78; 95% CI, 0.70–0.88; P=0.0001) than for chemotherapy versus surgery alone (HR 0.87; 95% CI, 0.79–0.96; P=0.005). However, no significant benefit in all-cause mortality for either chemoradiotherapy or chemotherapy could be observed by indirect comparison between the two regimens (HR 0.88; 95% CI, 0.76–1.01; P=0.07). A more recent meta-analysis that solely included clinical trials directly comparing neoadjuvant chemotherapy versus nCRT included six studies concerning 866 patients with esophageal or gastroesophageal adeno- or squamous cell cancer (43). This study reported a benefit of nCRT over chemotherapy in 3- and 5-year OS (RR 0.78, 95% CI, 0.62–0.98, P=0.03; RR 0.69, 95% CI, 0.50–0.96, P=0.03, respectively), R0 resection rate (RR 0.87, 95% CI, 0.81–0.92, P≤0.0001) and pathologically complete response rate (RR 0.16, 95% CI, 0.09–0.28, P<0.00001). This meta-analysis included mostly studies with a small sample size. Furthermore, the heterogeneity between studies was considered high and the earliest study that was included was published in 1992, solely randomized patients staged T1-2NxM0 and included patients between 1983 and 1988.
A retrospective multicenter propensity-score matched study aimed to compare OS in patients with esophageal adenocarcinoma undergoing either nCRT or chemotherapy (44). Between 2001–2012, 608 patients were included. After propensity-score matching, no differences in 3-year OS (57.9% versus 53.4%, P=0.391) nor in DFS (52.9% versus 48.9%, P=0.443) were reported in patients undergoing nCRT or chemotherapy, respectively. However, utilization of nCRT significantly increased incidence of ypT0 (26.7% versus 5%, P=0.001), ypN0 (63.3% versus 32.1%, P≤0.001) and significantly reduced R1/2 resection margins (7.7% versus 21.8%, P≤0.001).
Neither of the previously mentioned studies directly compared the chemotherapy regimens according to MAGIC, OEO2 or FLOT versus nCRT according to CROSS, although these are the most widely used regimens. Currently, several randomized controlled trials are addressing this topic. In the Neo-AEGIS trial, patients with adenocarcinoma of the esophagus or gastroesophageal junction are randomized between pre- and postoperative chemotherapy according to the MAGIC-regimen or FLOT-regimen versus nCRT according to the CROSS-regimen (45). This study aims at recruiting 594 patients and will be sufficiently powered to detect a 10% difference in favor of CROSS with a power of 80% and a significance of 5%. The primary endpoint of this study is OS. The ESOPEC-trial is a phase III two-arm trial that randomizes patients with adenocarcinoma of the esophagus or gastroesophageal junction between perioperative chemotherapy according to the FLOT-regimen followed by surgery versus nCRT according to the CROSS-regimen followed by surgery (46). This trial aims at including 438 patients at 16 centers. The primary aim of the study is OS and is calculated to detect a superiority in OS of the FLOT-regimen over the CROSS-regimen with a power of 80% and a significance of 5%. The NeXT-trial is a trial with a three-arm design that aims to include 501 with squamous cell carcinoma of the thoracic esophagus, with OS as primary endpoint (47). Patients are randomized between two 3-weekly courses of preoperative cisplatin (80 mg/m2) on day 1 with 5-fluorouracil (800 mg/m2) on days 1–5 or three 3-weekly courses of cisplatin (70 mg/m2) on day 1 with 5-fluorouracil (750 mg/m2) on days 1–5 and docetaxel (70 mg/m2) on day 1 or 41.4 Gy radiotherapy in 23 fractions with two 4-weekly courses of cisplatin (75 mg/m2) on day 1 with 5-fluorouracil (1,000 mg/m2) on days 1–5. With an expected increase of 10% in 3-year survival for preoperative DCF or RT-CF compared to CF alone, this study has a power of 70% with a significance of 5%.
Monoclonal antibodies
In the medical treatment of esophageal cancer patients, also immune-based therapies have been explored consisting of, among others, administration of monoclonal antibodies (mAbs). mAbs are known for their recognition of a specific DNA-sequence of a single epitope (i.e., the part of an antigen that is recognized by the antibodies). This potentially results in highly selective inhibition of molecular pathways or in enhanced response of a patient’s own immune system resulting in elimination of tumor cells (48). In order to diminish immune responses against mAbs, Riechmann et al. succeeded in ‘humanizing’ the monoclonal antibodies in 1988, by modifying the DNA in human antibodies in such a way that antibody regions of interest of, for example mice, are incorporated in the human antibody (49). This paved the way for widespread use of mAbs in human research and eventually lead to the first FDA-approval for usage of mAbs in the treatment of solid tumors in 1999. Single-agent administration of trastuzumab in patients with metastatic breast cancer resulted in durable objective responses and the side-effects were mostly mild to moderate (50). Trastuzumab is an antibody that binds and inhibits the Human Epidermal growth factor Receptor 2 (HER2/neu) which is expressed by the proto oncogene HER2/neu and is responsible for proliferation and inhibition of apoptosis of the cell. Subsequently, the publication from Bonner et al., reported an improvement of locoregional control and a reduction in mortality after addition of cetuximab to radiotherapy in patients with head and neck squamous cell carcinoma. This resulted in the FDA-approval of cetuximab, which binds and inhibits Epidermal Growth Factor Receptor (EGFR) and has similar functions as HER2/neu (51).
Currently two mAbs, ramucirumab and trastuzumab, are used in the clinical practice for the treatment of advanced upper-GI cancers. Ramucirumab inhibits angiogenesis by blockage of the vascular endothelial growth factor (VEGF)-receptor in regions where this receptor is overexpressed, mostly on tumor cells. Ramucirumab became part of clinical practice mainly after publication of the results of the REGARD-study that randomized 355 patients with gastric or gastroesophageal junctional adenocarcinoma who had disease progression after first-line platinum-containing or fluoropyrimidine-containing chemotherapy between ramucirumab monotherapy or a placebo (52). The median OS significantly improved from 3.8 months to 5.2 months after the addition of ramucirumab (HR 0.776; 95% CI, 0.603–0.998, P=0.047), while OS at 6 months improved from 31.6% to 41.8% and at 12 months from 11.8% to 17.6%.
Trastuzumab was incorporated in clinical practice mainly based on the study by Bang et al. (53). This study randomized 594 patients with advanced gastric or gastroesophageal junctional cancer between trastuzumab plus chemotherapy versus chemotherapy alone. The median follow-up was 18.6 months for patients undergoing trastuzumab and chemotherapy versus 17.1 months for patients undergoing chemotherapy alone. Median OS significantly improved from 11.1 months to 13.8 months after the addition of cetuximab (HR 0.74; 95% CI, 0.60–0.91, P=0.0046).
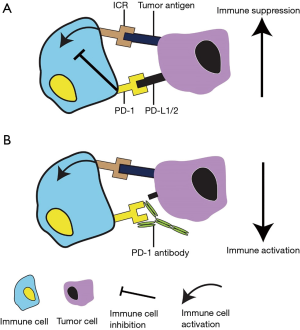
Several studies have been performed administrating monoclonal antibodies for treatment of esophageal cancer mainly using nivolumab, pembrolizumab or cetuximab (54-56). Both nivolumab and pembrolizumab are immune checkpoint inhibitors that block programmed cell death protein (PD)-1 expressed on immune cells. Normally, PD-1 has an immunoregulatory role in the immune system’s response to the cells of the human body with help of its ligands, by downregulation of the immune system and promoting self-tolerance (57). These ligands consist of PD-Ligand 1 (PD-L1) and PD-L2 and are often overexpressed on esophageal cancer cells (43.9%), resulting in an immune suppressive effect, preventing the immune system to attack tumor cells (58). By blockage of PD-1 using nivolumab or pembrolizumab, an immune suppressive effect by its ligands can be avoided and thus, the immune system will be better able to eliminate tumor cells. Consequently, blockage of PD-1 could result in immune related adverse events (Figure 1) (59). A phase II study by Kudo et al. administered nivolumab to 65 patients with esophageal squamous cell carcinoma that did not respond to, or were intolerant to fluoropyrimidine-based, platinum-based and taxane-based chemotherapy (55). After a median follow-up of 10.8 months, 17% had an objective response and the highest grade 3 and 4 adverse events were lung infection (8%) and dyspnoea or hyponatraemia (2%), respectively. Following this phase II study, a phase III study is currently randomizing patients with unresectable advanced or recurrent esophageal cancer between nivolumab monotherapy or docetaxel (75 mg/m2) every two weeks in combination with paclitaxel (100 mg/m2) weekly for six weeks until documented disease progression; the primary outcome of this study is OS (60). The estimated completion date of this study is September 2019. The KEYNOTE-590 study is currently investigating treatment of advanced or metastatic esophageal cancer by inhibition of PD-1 (56).This randomized, double-blind, placebo-controlled phase III trial aims to randomize 700 patients between cisplatin (80 mg/m2) every three weeks, 5-fluorouracil (800 mg/m2/day) via continuous infusion on days 1 to 5 in combination with either pembrolizumab or placebo. Primary outcomes of this study are progression-free survival and OS with subanalyses for PD-L1 positive patients. The estimated completion date of the study is planned in August 2021.
Only cetuximab has currently been tested in resectable esophageal cancer patients. The Swiss Group for Clinical Cancer Research (SAKK) has performed several studies using cetuximab in potentially curative esophageal cancer treatment. First, the phase Ib/II-SAKK 75/06 trial indicated that cetuximab could be safely added to induction chemotherapy followed by chemoradiotherapy showing high response rates and no increase in toxicity (41). Subsequently, a phase III trial was initiated (54,61). Between 2010 and 2013, 300 patients were included. The study randomized between two cycles of docetaxel (75 mg/m2) with cisplatin (75 mg/m2) on days 1 and 22 followed by chemoradiation consisting of 5 weekly cycles with intravenous docetaxel (20 mg/m2) and cisplatin (25 mg/m2) administered weekly for 5 weeks with concurrent 45 Gy in 25 fractions followed by surgery either with or without neoadjuvant and adjuvant cetuximab treatment. Neoadjuvant cetuximab consisted of 250 mg/m2 administered weekly during induction chemotherapy and during chemoradiotherapy, adjuvant treatment consisted of 500 mg/m2 every two weeks for three months. This resulted in a pathologically complete response rate for patients undergoing cetuximab of 37% (versus 33% in the control group). After a median follow-up of 4.0 years, median OS was 5.1 years and 3.0 years for the cetuximab and control group, respectively (HR 0.73; 95% CI, 0.52–1.10, P=0.055) with 5-year OS rates of 56% and 43%. For patients undergoing cetuximab, time to locoregional failure after R0-resection was significantly longer (HR 0.53; 95% CI, 0.31–0.90, P=0.017). However, systemic effects of addition of cetuximab seemed modest since time to distant failure did not differ between the two arms (HR 1.01; 95% CI, 0.64–1.59). Furthermore, one needs to realize that earlier studies of addition of cetuximab to definitive chemoradiotherapy failed to show a benefit in the nonoperative treatment of esophageal cancer (62-64). Given the limited data concerning the use of mAbs in the treatment of intentionally curative and resectable esophageal cancer, its value as part of the (neo)adjuvant treatment remains unclear.
Conclusions
Both chemotherapy and chemoradiotherapy have been adopted in the neoadjuvant armamentarium of potentially curative esophageal cancer, mainly based on the MAGIC-, OEO2- and CROSS-trials. The 5-year OS-advantage in the MAGIC- and OEO2-trials was 13% and 6%, respectively, compared to 14% in the CROSS-trial. The results of the FLOT-trial may change the landscape in chemotherapy treatment of esophageal cancer. Several studies, mostly retrospective, compared chemotherapy and chemoradiotherapy treatment in esophageal cancer patients. The results of these studies suggest a benefit for chemoradiotherapy in the number of pCR, R0-resections and possibly even in OS. The proposed higher rates of pCR after nCRT suggest that nCRT is more appropriate for a potential organ-sparing therapy in esophageal cancer, which has extensively been topic of debate. Results of large randomized clinical trials have to be awaited before a definitive answer can be given on the survival benefits in one of the two treatments. Furthermore, only cetuximab has been tested in the neoadjuvant setting and suggested a trend towards a better OS, a statistically significant improvement in locoregional recurrence and higher rates of pathologically complete response in one study. This is accompanied, however, by several other studies that failed to show benefit of cetuximab addition to definitive non-operative treatments for esophageal cancer. The currently applied neoadjuvant treatment regimens only show modest systemic effects. This results in relatively high rates of distant progression after neoadjuvant treatment and (unbeneficial) surgery. Future studies should mainly focus on enhanced systemic disease control.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Earlam R, Cunha-Melo JR. Oesophogeal squamous cell carcinoms: II. A critical view of radiotherapy. Br J Surg 1980;67:457-61. [Crossref] [PubMed]
- Earlam R, Cunha-Melo JR. Oesophageal squamous cell carcinoma: I. A critical review of surgery. Br J Surg 1980;67:381-90. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Muller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1990;77:845-57. [Crossref] [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Cruet-Hennequart S, Glynn MT, Murillo LS, et al. Enhanced DNA-PK-mediated RPA2 hyperphosphorylation in DNA polymerase eta-deficient human cells treated with cisplatin and oxaliplatin. DNA Repair (Amst) 2008;7:582-96. [Crossref] [PubMed]
- Garcia P, Braguer D, Carles G, et al. Comparative effects of taxol and Taxotere on two different human carcinoma cell lines. Cancer Chemother Pharmacol 1994;34:335-43. [Crossref] [PubMed]
- Alvarez P, Marchal JA, Boulaiz H, et al. 5-Fluorouracil derivatives: a patent review. Expert Opin Ther Pat 2012;22:107-23. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. [Crossref] [PubMed]
- Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979-84. [Crossref] [PubMed]
- Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 2007;25:3719-25. [Crossref] [PubMed]
- Salah-Eddin A-B, Nils H, Harald S, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. J Clin Oncol 2017;35:abstr 4004.
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Koizumi W, Tanabe S, Saigenji K, et al. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer 2003;89:2207-12. [Crossref] [PubMed]
- Sakata Y, Ohtsu A, Horikoshi N, et al. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 1998;34:1715-20. [Crossref] [PubMed]
- Sugimachi K, Maehara Y, Horikoshi N, et al. An early phase II study of oral S-1, a newly developed 5-fluorouracil derivative for advanced and recurrent gastrointestinal cancers. The S-1 Gastrointestinal Cancer Study Group. Oncology 1999;57:202-10. [Crossref] [PubMed]
- Hayata K, Ojima T, Nakamori M, et al. Neoadjuvant Chemotherapy with Docetaxel, Cisplatin and S-1 for Resectable Advanced Esophageal Cancer. Anticancer Res 2018;38:5267-73. [Crossref] [PubMed]
- Fang M, Song T, Liang X, et al. Comparative study of cisplatin-based definitive concurrent chemoradiotherapy with S-1 versus paclitaxel for unresectable locally advanced esophageal squamous cell carcinoma. Oncotarget 2017;8:37080-90. [Crossref] [PubMed]
- El Sayed YM, Sadee W. Metabolic activation of ftorafur [R,S-1-(tetrahydro-2-furanyl)-5-fluorouracil]: the microsomal oxidative pathway. Biochem Pharmacol 1982;31:3006-8. [Crossref] [PubMed]
- Hirose T, Fujita K, Nishimura K, et al. Pharmacokinetics of S-1 and CYP2A6 genotype in Japanese patients with advanced cancer. Oncol Rep 2010;24:529-36. [Crossref] [PubMed]
- Ajani JA, Faust J, Ikeda K, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 2005;23:6957-65. [Crossref] [PubMed]
- Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 2010;28:1547-53. [Crossref] [PubMed]
- al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol 1997;15:277-84. [Crossref] [PubMed]
- Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593-8. [Crossref] [PubMed]
- Amorino GP, Freeman ML, Carbone DP, et al. Radiopotentiation by the oral platinum agent, JM216: role of repair inhibition. Int J Radiat Oncol Biol Phys 1999;44:399-405. [Crossref] [PubMed]
- Servidei T, Ferlini C, Riccardi A, et al. The novel trinuclear platinum complex BBR3464 induces a cellular response different from cisplatin. Eur J Cancer 2001;37:930-8. [Crossref] [PubMed]
- Yang LX, Douple EB, Wang HJ. Irradiation enhances cellular uptake of carboplatin. Int J Radiat Oncol Biol Phys 1995;33:641-6. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Noordman BJ, Verdam MGE, Lagarde SM, et al. Effect of Neoadjuvant Chemoradiotherapy on Health-Related Quality of Life in Esophageal or Junctional Cancer: Results From the Randomized CROSS Trial. J Clin Oncol 2018;36:268-75. [Crossref] [PubMed]
- Noordman BJ, Verdam MGE, Lagarde SM, et al. Impact of neoadjuvant chemoradiotherapy on health-related quality of life in long-term survivors of esophageal or junctional cancer: results from the randomized CROSS trial. Ann Oncol 2018;29:445-51. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Conroy T, Etienne PL, Adenis A, et al. Vinorelbine and cisplatin in metastatic squamous cell carcinoma of the oesophagus: response, toxicity, quality of life and survival. Ann Oncol 2002;13:721-9. [Crossref] [PubMed]
- Ajani JA, Xiao L, Roth JA, et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2013;24:2844-9. [Crossref] [PubMed]
- Shimodaira Y, Slack RS, Harada K, et al. Influence of induction chemotherapy in trimodality therapy-eligible oesophageal cancer patients: secondary analysis of a randomised trial. Br J Cancer 2018;118:331-7. [Crossref] [PubMed]
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Zhao X, Ren Y, Hu Y, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: A meta-analysis based on clinical trials. PLoS One 2018;13:e0202185. [Crossref] [PubMed]
- Markar SR, Noordman BJ, Mackenzie H, et al. Multimodality treatment for esophageal adenocarcinoma: multi-center propensity-score matched study. Ann Oncol 2017;28:519-27. [PubMed]
- Reynolds JV, Preston SR, O'Neill B, et al. ICORG 10-14: NEOadjuvant trial in Adenocarcinoma of the oEsophagus and oesophagoGastric junction International Study (Neo-AEGIS). BMC Cancer 2017;17:401. [Crossref] [PubMed]
- Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016;16:503. [Crossref] [PubMed]
- Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 2013;43:752-5. [Crossref] [PubMed]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975;256:495-7. [Crossref] [PubMed]
- Riechmann L, Clark M, Waldmann H, et al. Reshaping human antibodies for therapy. Nature 1988;332:323-7. [Crossref] [PubMed]
- Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17:2639-48. [Crossref] [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Ruhstaller T, Pless M, Dietrich D, et al. Cetuximab in combination with chemoradiotherapy before surgery in patients with resectable, locally advanced esophageal carcinoma: a prospective, multicenter phase IB/II Trial (SAKK 75/06). J Clin Oncol 2011;29:626-31. [Crossref] [PubMed]
- Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017;18:631-9. [Crossref] [PubMed]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT00287391, First-line Esophageal Carcinoma Study With Chemo vs. Chemo Plus Pembrolizumab (MK-3475-590/KEYNOTE-590); 2017 Jun 16 [cited 2018 Oct 22];. Available online: https://clinicaltrials.gov/ct2/show/NCT03189719
- Sharpe AH, Wherry EJ, Ahmed R, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007;8:239-45. [Crossref] [PubMed]
- Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53. [Crossref] [PubMed]
- Wang DY, Johnson DB, Davis EJ. Toxicities Associated With PD-1/PD-L1 Blockade. Cancer J 2018;24:36-40. [Crossref] [PubMed]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT00287391, Study of Nivolumab in Unresectable Advanced or Recurrent Esophageal Cancer.; 2015 Oct 6 [cited 2018 Oct 22];. Available online: https://clinicaltrials.gov/ct2/show/NCT02569242
- Ruhstaller T, Thuss-Patience P, Hayoz S, et al. Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: a randomized, open-label, phase III trial (SAKK 75/08). Ann Oncol 2018;29:1386-93. [Crossref] [PubMed]
- Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol 2013;14:627-37. [Crossref] [PubMed]
- Crosby T, Hurt CN, Falk S, et al. Long-term results and recurrence patterns from SCOPE-1: a phase II/III randomised trial of definitive chemoradiotherapy +/- cetuximab in oesophageal cancer. Br J Cancer 2017;116:709-16. [Crossref] [PubMed]
- Suntharalingam M, Winter K, Ilson D, et al. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients With Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial. JAMA Oncol 2017;3:1520-8. [Crossref] [PubMed]


