
Ultimate management of post thoracotomy morbidities: a set of surgical technique and peri-operative precautions
Introduction
Thoracotomy is the traditional approach to gain pleural cavity in thoracic surgery. Its feature is chest wall opening by costal spreading to provide a direct and wide surgical field view. It allows to comfortably access the throat, lungs, heart and diaphragm. Thoracotomy is not only a classic approach to pleural space but it is also the most common so far. Despite the advent of minimally invasive surgery, that over the last two decades has established itself as the preferred approach for almost all thoracic surgical procedures in high specialized centers (1), thoracotomy indeed is still recommended for many challenging surgical interventions.
Therefore, when thoracotomy should be preferred to video-assisted thoracoscopic surgery (VATS) and which is the best thoracotomy for a fast recovery are definitively ongoing discussion topics among thoracic surgeons.
There are many different surgical approaches to performing a thoracotomy, mainly differing in what muscle group is dissected and extent of costal spreading.
Each thoracotomy type allows different exposure of the surgical field and surgeons are supposed to adopt the best one case by case. However, pleural cavity exposure is not the only criteria to choose a thoracotomy but morbidities and the impact on patient recovery are meaningful too.
To overcome thoracotomy side-effects, during the last decades many modified procedures have been introduced with the aim to minimally damage chest wall by reducing skin incision and sparing thoracic muscles.
Posterolateral versus muscle sparing thoracotomy (MST)
Thoracotomy is related to many morbidities; the most frequent and feared are acute and chronic post-thoracotomy pain, impaired respiratory function and shoulder movements reduction.
Posterolateral thoracotomy (PLT) has been the preferred access for almost every thoracic surgeon during the last century. It guarantees good access to pulmonary hilum and mediastinum and can be quickly enlarged in case of intra-operative emergency. However, it is characterized by the division of latissimus dorsi (LD) and serrates anterior (SA) and sometimes trapezius, rhomboids and paravertebral muscles. As a result, this procedure is considered highly impacting on patient’s postoperative recovery. The International Association for the Study of Pain reported prevalence of post thoracotomy pain up to 80% of cases, with 50% presenting significant discomfort even 1 year after surgery. According to these results, PLT seems to be very badly influencing on patient’s quality of life (QoL).
With the aim to reduce post thoracotomy complications, many other procedures preserving major muscle groups have been proposed. These are known as MST or nerve sparing thoracotomy (NST) depending on the performed technique. They avoid any dissection of LD and preserve SA by the division of its fibers in line with their direction. The most diffuse MST are the vertical axillary described by Browne in 1953 (2), the lateral proposed by Mitchell et al. (3) and the posterior with muscle preserving technique proposed by Bethencourt et al. (4). Then, in the last decades these procedures have been improved in order to further minimize muscle injury. The introduction of MST was based on the conviction to reduce postoperative pain, preserve pulmonary function and reduce postoperative complication. However, the question if purpose has been reached is still unresolved.
One of the most recent meta-analysis comparing outcomes of MST and PLT has been published by Uzzaman et al. in 2014 (5). They included 12 trials from 1991 to 2010, with a population of 1.083 patients. Primary outcomes were lung function tests, shoulder movements and postoperative pain. Supported by a solid statistical analysis, despite few biases, results can be resumed in 3 points: (I) pain score on day 1 and 30 was not reduced significantly in MST. Reduction of pain was significantly in favor of MST, only on day 7; (II) any significant difference in forced expiratory volume-one second (FEV1) and vital capacity (VC) impairment after 30 days was registered; (III) arm internal rotation was significantly improved in MST.
Considering that MST should be advocated to highly reduce post thoracotomy morbidities, this meta-analysis results are unsatisfying, especially as concerning pain and pulmonary function.
According to Authors, pain score on day 1 could have been similar in the two groups because of the contribution to post thoracotomy pain of many factors such as skin incision, ribs spreading, intercostal nerve compression or of aggressive postoperative analgesics administration.
The lack of significant difference in pulmonary dysfunctions could be due to absence of difference in pain score itself and to the weak role of LD and SA in respiration.
Hence, Authors conclusions moderately support the adoption of MST approach, especially in physically active patients, since associated with better outcomes as regards shoulder movement and postoperative pain relief. However, the advantages of MTS are not enough remarkable to conclude that PLT should be quit and that post thoracotomy morbidities have been definitively addressed.
NST, a further innovation
Since MST seems to be not quite successful in reducing postoperative morbidities, some authors have proposed a technique to spare also intercostal nerves. The rationale arises from many neurophysiologic studies showing an association between postoperative pain intensity and intercostal nerve damage. During thoracotomy two neurovascular bundles are injured; the bundle of the rib above is pressed by retractor vertical blade during almost all the intervention, whereas the bundle of the rib below is squeezed by pericostal closure suture.
Therefore, NST should overcome any nerves injuries by avoiding intercostal suture and rib spreader pressure. NST preserve the above neurovascular pedicle dissecting the intercostal muscle flap and the below performing an intracostal suture by drilling the inferior rib.
Jiwnani et al., in 2018 (6) have published a randomized trial comparing pain after NST versus PLT. Their outcomes were assessment of pain and adverse events in a population of ninety randomized patients. Unfortunately, results were unsatisfying since Author’s modified thoracotomy did not provide any advantage in terms of pain reduction and all outcomes compared, including drug consumption and chronic pain, were similar in the two groups.
They also reported a short literature review to underline that, despite some papers were able to find a significant pain reduction when intracoastal suture or intercostal muscle flap were adopted, there is not an evidence that NST provide lower postoperative complication than classic PLT. In fact, many criticisms of these studies have been underlined, such as small population enrolled or lack of patient and assessor blinding.
Therefore, they concluded that even an advanced surgical technique as NST does not affect post thoracotomy morbidities. We truly appreciated their final comment underlining that pain intensity after thoracotomy is influenced by several factors other than thoracotomy. These could be intra-operative as operative time and thoracostomy for chest tube or postoperative factors as in particular analgesic technique.
We congratulate the Authors because with their paper they have point out that a multidisciplinary approach to post thoracotomy morbidities is needed. From their conclusions we learn that the best thoracotomy procedure for the whole of patients does not exist. On the contrary, surgeons should select the optimal approach for each patient based on surgical field exposure required and include any multidisciplinary precautions for postoperative management.
Anyway, Jiwnani’s paper is not the first study comparing PLT versus NST. In 2014 Celikten (7) and co-workers presented a similar study with consistent conclusions and, in 2012, Elshiekh et al. (8), presented a best evidence topic set as a protocol as reported in IVCTS (9). Conclusions were based on a selection of prospective randomized studies and suggested that MST better preserve muscle strength and arm motion but only in the first postoperative week. Conversely, there was no evidence of difference in pain score and pulmonary function impairment on the thoracotomy type.
Our thinking, based on literature and on personal experience is that MST and NST are effective procedures alternative to PLT, since generally provide some advantages in post thoracotomy morbidities reduction. However, there is not an impressive superiority over PLT and, when wider exposure is needed, PLT should be always considered without worry to significantly worsen patient recovery.
Role of analgesia in conditioning post thoracotomy pain and pulmonary function
Pain is the most important factor responsible for postoperative pulmonary function impairment in thoracic surgery (10). Due to a postoperatively restrictive respiratory pathway development, the baseline values of FEV1, forced vital capacity (FVC), and functional residual capacity (FRC) usually decrease to about 40%. Atelectasis is conditioned by the relationship between functional residual lung capacity and closing capacity; when functional residual lung capacity goes down to a value inferior to closing capacity, the airways start narrowing producing an area with low ventilation/perfusion rate. At the same time, pain highly reduces voluntary deep breathing, coughing, and patient mobility, all needed to break down atelectasis.
Surgical approach is the main issue determining postoperative pain nature and intensity. It has been showed that PLT is more painful than limited thoracotomy and VATS during the first week (11). In detail, the posterior spinal muscles retraction determines stimuli delivered by the posterior primary branches of the spine, while incision pain is addressed by the anterior branches. Moreover, pain from visceral pleura, lung, and neuro-humoral factors is mediated by sympathetic nerves. All of these pathways play a role in pain occurrence and must be stopped to manage it.
Many approaches for the management of post-thoracotomy pain are known. Intravenous narcotic drugs may properly succeed, but are affected by cough suppression, as well as central nervous system and respiratory depression, determining once more retention of secretions and atelectasis. To avoid systemic narcotics side effects, EA has been declared the gold standard for pain management during and after open thoracic surgery (12,13). It allows direct anesthetization of the spinal branches and sympathetic nerves, less narcotic drugs use, better pain control and decreased respiratory and central nervous system impairment (14). However, it presents several absolute (e.g., coagulopathy, local sepsis, allergy to amide local anesthetics, and anatomical anomalies) and relative (activated partial thromboplastin time ratio or international normalized ratio 1.2–1.4) contraindications (15). Moreover, some insertion technical troubles have been reported up to 11% of patients. Bilateral effects of local anesthetic on the sympathetic chain have been associated with increasing hypotension, itching, vomiting/nausea, urinary retention, and sometimes mental status changes and respiratory impairment have been described.
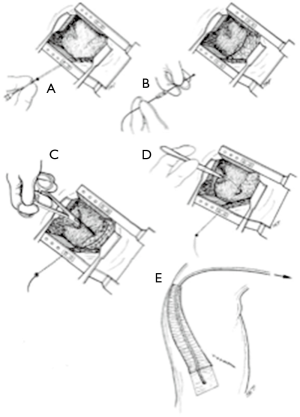
In 2013 we presented a prospective randomized study comparing EP with analgesic continuous infusion by paravertebral catheter (PC) (16) (Figure 1). Our results were significantly in favor of paravertebral analgesia (PA). Firstly, we found significant advantages concerning pain management, both at rest and while coughing, at 72 hours after surgery. Second, PA was more effective than EA to preserve pulmonary function in terms of FEV1 (P=0.023) and ambient air saturation (P=0.023). As regards catheter placement, PA was more effective. It is a safe and easy to learn procedure performed by surgeons, usually at the end of intervention, whereas patients are still under anesthesia. It takes about 5 minutes, reducing the overall perioperative time. Moreover, PA is suitable also for patients who are referred to VATS, without EA catheter, but then are converted to thoracotomy during surgery, no matter the reason. PA does not present contraindications in case of coagulopathies or anatomical anomalies and is not affected by systemic collateral effects thanks to the use of local anesthetics. Lastly, our results clearly showed the absence of adverse effects in PA group suggesting its lower danger. So, we concluded that PA is safe and effective and should be always considered in alternative to EA catheter.
QoL after thoracotomy
Patients reported outcomes (PROs) concerning QoL, have been found to correlate to postoperative outcomes, and also to survival in lung cancer patients (17). Furthermore, QoL has been introduced into government strategic frameworks to be supported by hospitals with the aim to allocate financial bonus or research grants.
QoL is worsen by any affliction determining negative influences on patient’s physical, social and mental health. It is common opinion, supported by some papers (18) that thoracotomy adversely affected also QoL, in addition to morbidities addressed above, but any evidence is lacking, since PROs collection is particularly difficult.
In 2017, Schwartz et al. (19), presented their study with the aim to better understand the influence of different type of thoracic access on postoperative patients’ QoL. Surprising, they concluded that QoL variation was similar in VATS and thoracotomy for early stage lung cancer patients. Also, Salati et al. (20) showed that at 3 months, both thoracotomy and VATS patients presented similar lowering in preoperative functional parameters; moreover, VATS lobectomy did not provide any benefit in terms of FEV1, carbon monoxide diffusing capacity (DLCO) and exercise capacity recovery over MST.
However, if we focus on papers comparing QoL exclusively between different types of thoracotomies, few data are present in literature.
An interesting series has been published by Alar et al. in 2014, comparing various types of thoracotomy using the Short Form-36 Health Survey (21). They showed that the standard PLT strongly supports adverse effect on QoL compared to MST, especially due to dissecting the LD muscle. Unfortunately, this article is unique and there are too small data to support any kind of evidence. Nowadays, patients firstly and firmly aspire to complete healing, but are very interested in daily postoperative QoL as well. Therefore, in the last decade, the attention in QoL evaluation has developed at the same rate than investigations on survival and morbidities.
In our opinion QoL must be an additional parameter to consider evaluating different surgical procedures, since clinical outcomes alone are no more satisfactory in modern medicine. This is way further studies are desirable on this significant issue.
Conclusions
The advent of minimally invasive thoracic surgery in the last two decades has focused all the attentions to the comparison of outcomes between VATS and open surgery. Beyond the oncological outcomes, the main topic of this comparison was the incidence of post-operative morbidities. Based on several studies, it has been showed that, in the very first postoperative period, VATS guarantee better outcomes in terms of pain score and pulmonary function impairment.
However, the role of open thoracotomy has not been exceeded in clinical practice. Therefore, we found that the paper by Jiwnani et al. is an extremely present-day study. Despite they presented an innovative and advanced NST type, results were consistent with literature confirming that usually MST and NST are slightly better than PLT.
This confirm that the best strategy to manage open thoracic surgery and its preoperative complications is a multifactorial matter. We think that MST and NST are more advisable than PLT in routine practice. But, when a wider surgical field is needed, there is no reason to hesitate performing a classic PLT. Moreover, the best management of post-operative morbidities does not result only from the procedure type but from intra and post-operative analgesia adopted. In particular we think that PA should be considered as the first choice in this kind of patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cao C, Frick AE, Ilonen I, et al. European questionnaire on the clinical use of video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2018;27:379-83. [Crossref] [PubMed]
- Singh SJ, Kapila L. Denis Browne’s thoracotomy revised. Pediatr Surg Int 2002;18:90-2. [Crossref] [PubMed]
- Mitchell R, Angell W, Wuerflein R, et al. Simplified lateral chest incision for most thoracotomies other than sternotomy. Ann Thorac Surg 1976;22:284-6. [Crossref] [PubMed]
- Bethencourt DM, Holmes EC. Muscle-sparing posterolateral thoracotomy. Ann Thorac Surg 1988;45:337-9. [Crossref] [PubMed]
- Uzzaman MM, Robb JD, Mhandu PC, et al. A meta-analysis comparing muscle-sparing and posterolateral thoracotomy. Ann Thorac Surg 2014;97:1093-102. [Crossref] [PubMed]
- Jiwnani S, Ranganathan P, Patil V, et al. Pain after posterolateral versus nerve-sparing thoracotomy: A randomized trial. J Thorac Cardiovasc Surg 2019;157:380-6. [Crossref] [PubMed]
- Celikten A, Sayar A, Metin M, et al. Anterior muscle and neurovascular-sparing thoracotomy and posterolateral thoracotomy: postoperative pain and morbidity assessment. Thorac Cardiovasc Surg 2014;62:353-6. [Crossref] [PubMed]
- Elshiekh MA, Lo TT, Shipolini AR, et al. Does muscle-sparing thoracotomy as opposed to posterolateral thoracotomy result in better recovery? Interact Cardiovasc Thorac Surg 2013;16:60-7. [Crossref] [PubMed]
- Dunning J, Prendergast B, Mackway-Jones K. Towards evidence-based medicine in cardiothoracic surgery: best BETS. Interact Cardiovasc Thorac Surg 2003;2:405-9. [Crossref] [PubMed]
- Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003;362:1921-8. [Crossref] [PubMed]
- Landreneau RJ, Pigula F, Luketich JD, et al. Acute and chronic morbidity differences between muscle-sparing and standard lateral thoracotomies. J Thorac Cardiovasc Surg 1996;112:1346-50; discussion 1350-1. [Crossref] [PubMed]
- Kaiser AM, Zollinger A, De Lorenzi D, et al. Prospective, randomized comparison of extrapleural versus epidural analgesia for postthoracotomy pain. Ann Thorac Surg 1998;66:367-72. [Crossref] [PubMed]
- Bakhtiary F, Therapidis P, Dzemali O, et al. Impact of high thoracic epidural anesthesia on incidence of perioperative atrial fibrillation in off-pump coronary bypass grafting: a prospective randomized study. J Thorac Cardiovasc Surg 2007;134:460-4. [Crossref] [PubMed]
- Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg 2009;36:170-80. [Crossref] [PubMed]
- Hyllested M, Jones S, Pedersen JL, et al. Comparative effect of paracetamol, NSAIDs or their combination in postoperative pain management: a qualitative review. Br J Anaesth 2002;88:199-214. [Crossref] [PubMed]
- Raveglia F, Rizzi A, Leporati A, et al. Analgesia in patients undergoing thoracotomy: epidural versus paravertebral technique. A randomized, double-blind, prospective study. J Thorac Cardiovasc Surg 2014;147:469-73. [Crossref] [PubMed]
- Pompili C, Novoa N, Balduyck B, et al. Clinical evaluation of quality of life: a survey among members of European Society of Thoracic Surgeons (ESTS). Interact Cardiovasc Thorac Surg 2015;21:415-9. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Schwartz RM, Yip R, Flores RM, et al. The impact of resection method and patient factors on quality of life among stage IA non-small cell lung cancer surgical patients. J Surg Oncol 2017;115:173-80. [Crossref] [PubMed]
- Salati M, Brunelli A, Xiumè F, et al. Video-assisted thoracic surgery lobectomy does not offer any functional recovery advantage in comparison to the open approach 3 months after the operation: a case matched analysis†. Eur J Cardiothorac Surg 2017;51:1177-82. [Crossref] [PubMed]
- Alar T, Ceylan KC, Kaya SO, et al. How does the type of thoracotomy affect the patient quality of life? A short form-36 health survey study. Surg Today 2014;44:264-70. [Crossref] [PubMed]


