
Learning curve and postoperative outcomes of minimally invasive esophagectomy
Introduction
Innovation is necessary to improve health care. The aim of implementing a surgical innovation is to increase surgical effectiveness and to decrease postoperative complications compared to older procedures. Patients increasingly expect to be operated by the newest techniques, which generally are complex and more difficult to learn. The introduction of surgical innovations, however, is associated with learning curves and learning curves may have a negative impact on patient outcome (1).
It is important to take surgical learning curves into account when interpreting outcome data that is acquired during an implementation period. In general, the significance of surgical learning curves is increasing, since the complexity of currently implemented surgical procedures is increasing and interventions are implemented at an increasing rate.
In addition to guiding the interpretation of outcome data during the implementation period, learning curve analysis is becoming increasingly important to expose differences in lengths of learning curves and learning associated morbidity (extra morbidity that occurs during the learning phase that could have been avoided if patients were operated by truly proficient surgical teams). Several studies have shown that learning curves of technically challenging procedures can take years to complete and results can be significantly impaired during this learning phase (2-5). In contemporary surgery, differences in effectiveness between newly implemented, innovative procedures are relatively small in general and therefore, impaired outcome during the learning phase is becoming relatively more significant. This may especially be the case for different types of minimally invasive esophagectomy (MIE). However, different types of MIE have not been compared directly regarding length of the learning curve or learning associated morbidity.
In this article, we aimed to review the results of studies that investigated the learning curve of MIE. Outcome parameters of interest were operative time, intraoperative blood loss and clinically more relevant parameters such as amount of retrieved lymph nodes, anastomotic leakage, overall morbidity, hospital length of stay and postoperative mortality.
Learning curve of MIE
Determining the length of the learning curve is important to inform clinicians about what they can expect after implementation of MIE. However, there is significant heterogeneity in methodology between learning curve studies and important differences exist between studies regarding outcome parameters, learning curve analysis methods and correction for casemix.
Most reports have used intraoperative variables, such as operative time (6-18), but other reports have also used clinically more relevant outcome parameters, such as postoperative complications and anastomotic leakage (13,14,19). Regarding analysis methods, most studies assigned patients to arbitrarily created groups and compared outcomes of patients operated on early after implementation between patients operated on later (9,11,20-27). Other studies used cumulative sum (CUSUM) analysis or variations of CUSUM and therefore omitted splitting a patient population in arbitrarily created groups (6,8,10,11,14,16,18,19). Casemix correction is only performed in few studies that investigated the learning curve of MIE (14).
Because of these differences in study methodology, it is difficult to compare results of different learning curve studies and this is further complicated by the fact that different approaches of MIE exist.
Learning curve of McKeown MIE
Twelve studies that conducted a learning curve analysis of only McKeown MIE have been published (Table 1). There were no studies that used pooled results of multiple surgeons in multiple hospitals. The number of included patients ranged from 28 to 237. Only 3 studies used CUSUM analysis to determine the length of the learning curve, the other 8 studies compared outcomes between arbitrarily divided groups of patients. The length of the learning curve ranged from 25 (11) to 175 (8) cases based on improved results of operative time (Table 1).
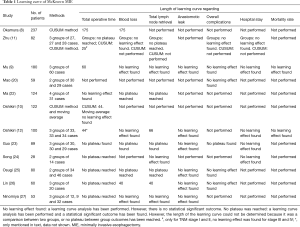
Full table
Nine studies investigated the length of the learning curve with postoperative complications as outcome parameter, but only three studies reported a decrease in postoperative complications with increased experience. Guo et al. (23) reported a decrease in overall complications from 53% in the first 30 patients to 7% in the last 29 patients (P=0.0005). Okamura et al. (8) found a decrease in pneumonia incidence from 18.9% during the learning curve, the first 175 cases, to 6.5% after the learning curve had been completed (P=0.024). Osugi et al. (25) also found a decrease in pulmonary infection between the first 34 patients and the last 46 patients (P=0.0127). Interestingly, all included studies reported anastomotic leakage rate, but none of the studies found a significant learning curve regarding anastomotic leakage.
Learning curve of Ivor Lewis MIE
There are four studies that conducted a learning curve analysis for Ivor Lewis MIE (Table 2). Only one study used pooled results of multiple hospitals (19) and the number of included patients ranged from 80 to 646. Two studies used the CUSUM method and one study used linear regression analysis to determine the length of the learning curve. The other study compared outcomes between two arbitrarily divided groups. Two of these studies (19,21) used anastomotic leak to determine the length of the learning curve, two used operative time.
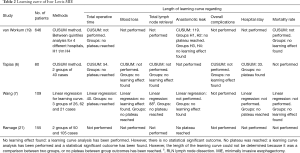
Full table
The length of the learning curve ranged from 40 (8) to 54 (6) based on operative time. Ramage et al. (21) who compared the first 50 patients with the subsequent 105 patients had gastric tube necrosis, anastomotic leak, and combined gastric tube necrosis and leak as outcome. They found a decrease from 18% to 7% (P=0.0457) and from 22% to 10% (P=0.0447) for anastomotic leak and combined gastric tube necrosis and leak rate, respectively. In our own learning curve study, that included 646 patients from 4 high volume hospitals, we used CUSUM analysis and found a length of the learning curve of 119 (19) cases based on anastomotic leak. We found a mean incidence of anastomotic leakage of 18.8% during the learning curve and 4.5% after the plateau had been reached after 119 cases. Using area under the curve analysis we concluded 36 patients (10.1% of all patients that were operated during the learning curve) experienced learning associated anastomotic leakage: anastomotic leakage that could have been prevented if the patients were operated by a surgeon who had completed the learning curve.
Learning curve of robot-assisted minimally invasive esophagectomy (RAMIE)
RAMIE is the latest surgical innovation for esophageal resection. Six studies have described a learning curve both for the Ivor Lewis procedure and the McKeown procedure (Table 3). There were no studies that used pooled results of multiple hospitals. The number of patients included in these studies ranged from 52 to 232. Three studies used CUSUM analysis to determine the length of the learning curve and the other three studies compared outcomes between arbitrarily divided groups.
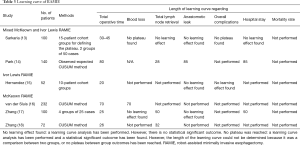
Full table
Sarkaria et al. (13) included both Ivor Lewis RAMIE and McKeown RAMIE and found a decrease in operative time between 30 to 45 procedures. They also noted a decrease in overall complications from 58% in the first 50 patients to 44% in the last 50 patients (P=0.046). Park et al. (14) who also included both Ivor Lewis RAMIE and McKeown RAMIE concluded the learning curve was completed after 80 cases based on operative time and 85 cases based on anastomotic leakage. In their study, anastomotic leakage rate decreased from 15% during the learning curve, to 2% after the learning curve had been completed.
Three studies only included McKeown RAMIE. van der Sluis et al. (16) concluded McKeown RAMIE could be performed proficiently after 70 procedures based on operative time, intraoperative blood loss and conversion rate. According to Zhang et al. (18) the length of the learning curve is only 26 cases based on operative time. This is consistent with Zhang et al. (17) who compared outcomes between four groups and found a plateau in operative time, and thus a completion of the learning curve, after 25 cases (P<0.001).
Hernandez et al. (15) only included Ivor Lewis RAMIE and found a decrease in operative time from 514 to 397 minutes (P<0.005) after 20 cases, but they did not find a learning curve regarding anastomotic leak.
Discussion of MIE learning curve study results
The relation between learning curves and postoperative morbidity
Some interesting observations can be made from the studies in this review. First, the included studies have reported a wide range of learning curve length from 20–175 cases regarding operative time, blood loss, harvested lymph nodes, hospital stay and postoperative complications. None of the reviewed studies found a learning curve regarding postoperative mortality. Second, studies that included more patients generally found a longer length of the learning curve for MIE. This is not surprising, since for example small study of 40 patients undergoing MIE can never establish that the learning curve is longer than 40 cases. This finding supports the opinion that the length of a learning curve that is found in a small case series should be interpreted with caution. Third, it is interesting that none of the studies that included patients undergoing McKeown MIE or McKeown RAMIE found a learning curve for anastomotic leakage, but a learning curve of 50–119 cases was found in three studies that included Ivor Lewis (RA)MIE. This can be explained by the fact that a cervical anastomosis is performed by open surgery, and surgeons that were learning McKeown MIE may have already been familiar with this anastomosis. This is in contrast to the minimally invasive creation of an intrathoracic anastomosis, where surgeons that were learning Ivor Lewis MIE also had to learn a new anastomotic technique. All studies that found a learning curve regarding anastomotic leakage after implementation of Ivor Lewis (RA)MIE, found that anastomotic leakage decreased by at least 10% during the learning curve phase. This means that a substantial extra number of patients may be at risk for anastomotic leakage during the learning curve, possibly with devastating sequelae. It may be sensible for surgeons who want to implement a MIE program, to start with a cervical anastomosis (McKeown procedure) and consider implementation of the Ivor Lewis MIE after other important skills have been learned.
Methodological considerations
Drawing general conclusions from the published MIE learning curve studies is challenging because differences in study methodology and the limited quality of most studies that have been performed. Most included studies are small, single center studies. These studies used different learning curve analysis methods. Thirteen studies assigned patients to groups of arbitrary size, which is susceptible to bias because authors can make groups that fit their data. Eight studies performed the CUSUM analysis. CUSUM analysis avoids splitting patients into groups by authors which results in a lower risk of bias. In addition, CUSUM analysis can be used to identify the length of the learning curve per patient instead of analyzing groups of patients, which can result in more precise estimations of the length of the learning curve. Correction for casemix is also important for learning curve analysis, since it is plausible that some surgeons expand indications to more complex cases with increasing experience. However, only 2 of the included studies took casemix into account in the analysis (14,19).
In addition, the experience levels of surgeons that performed the surgeries that were included in the learning curve studies varies widely and it is likely that this contributes to the wide range of learning curve lengths that were found. Most studies have described different levels of experience to some extent, but in order to establish a mean length of a learning curve for a procedure it may be better to analyze pooled data from multicenter datasets.
Safe implementation
With increasing complexity of surgical procedures, it is becoming more and more important to establish effective and safe implementation programs in order to reduce learning associated morbidity. Since some MIE learning curve studies have shown that morbidity can be significantly increased during learning curves (19,21), safe implementation programs could substantially improve outcome during learning curves. Efforts have been made to ensure safe implementation of new surgical techniques and these have for example consisted of (inter)national safe implementation guidelines. However, some of the learning curve studies that were reviewed for this manuscript were performed after safe implementation guidelines had already been established, suggesting that these guidelines have to be improved further to increase effectiveness and enhance patient safety during learning curves.
Various other methods exist that can support safe implementation programs. Video-based platforms can give feedback regarding performance that can be used for coaching and surgical quality improvement (28). Various training models exist, but there is no consensus on what factors contribute to effective training and there is no consensus regarding what short-term outcomes are most relevant (10,26). Ruurda et al. (29) suggested a structured training program for RAMIE that should enable surgeons with basic MIE skills and knowledge to complete the learning curve in 20 cases, but more research is necessary to verify if it is really feasible. Another widely used method for safe implementation is proctorship. Ninomiya et al. (27) have shown that an experienced surgeon can instruct surgeons at another institution and possibly shorten the learning curve of the new instructed surgeon. This is consistent with Oshikiri et al. (10), who compared the learning curve based on operative time between two surgeons. With surgeon A there was a decrease after the 44th case in operative time. Surgeon B implemented a stable standard procedure developed by surgeon A who also acted as a proctor to surgeon B. With surgeon B there was a decrease in operative time after the 17th case, thus a shorter learning curve.
In general, there is little robust evidence on what factors contribute to more effective learning and safe implementation of innovative and technically challenging surgical techniques.
Conclusions
The significance of surgical learning curves is increasing because recent surgical innovations are complex and therefore the length of the learning curve is longer. Surgical learning curves of MIE can be associated with significant morbidity and this has especially been established for Ivor Lewis MIE. Safe implementation programs are therefore increasingly important to diminish learning associated morbidity. More research is needed to develop evidence based safe implementation programs to ensure patient safety during learning curves.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12. [Crossref] [PubMed]
- Doumouras AG, Saleh F, Anvari S, et al. Mastery in bariatric surgery: the long-term surgeon learning curve of Roux-en-Y gastric bypass. Ann Surg 2018;267:489-94. [Crossref] [PubMed]
- Carandina S, Montana L, Danan M, et al. Laparoscopic Sleeve Gastrectomy Learning Curve: Clinical and Economical Impact. Obes Surg 2019;29:143-8. [Crossref] [PubMed]
- Mackenzie H, Markar SR, Askari A, et al. National proficiency-gain curves for minimally invasive gastrointestinal cancer surgery. Br J Surg 2016;103:88-96. [Crossref] [PubMed]
- Brown KM, Geller DA. What is the learning curve for laparoscopic hepatectomy? J Gastrointest Surg 2016;20:1065-71. [Crossref] [PubMed]
- Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg 2014;218:1130-40. [Crossref] [PubMed]
- Wang Q, Wu Z, Chen G, et al. Two-stage indicators to assess learning curves for minimally invasive Ivor Lewis esophagectomy. Thorac Cardiovasc Surg 2018;66:362-9. [Crossref] [PubMed]
- Okamura A, Watanabe M, Fukodome I, et al. Surgical team proficiency in minimally invasive esophagectomy is related to case volume and improves patient outcomes. Esophagus 2018;15:115-21. [Crossref] [PubMed]
- Mu JW, Gao SG, Xue Q, et al. Updated experiences with minimally invasive McKeown esophagectomy for esophageal cancer. World J Gastroenterol 2015;21:12873-81. [Crossref] [PubMed]
- Oshikiri T, Yasuda T, Yamamoto M, et al. Trainee competence in thoracoscopic esophagectomy in the prone position: evaluation using cumulative sum techniques. Langenbecks Arch Surg 2016;401:797-804. [Crossref] [PubMed]
- Zhu ZY, Yong X, Luo RJ, et al. Clinical analysis of minimally invasive McKeown esophagectomy in a single center by a single medical Group. J Zhejiang Univ Sci B 2018;19:718-25. [Crossref] [PubMed]
- Oshikiri T, Yasuda T, Hasegawa H, et al. Short-term outcomes and one surgeon’s learning curve for thoracoscopic esophagectomy performed with the patient in the prone position. Surg Today 2017;47:313-9. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Grosser R, et al. Attaining proficiency in robotic-assisted minimally invasive esophagectomy while maximizing safety during procedure development. Innovations (Phila) 2016;11:268-73. [PubMed]
- Park S, Hyun K, Lee HJ, et al. A study of the learning curve for robotic oesophagectomy for oesophageal cancer. Eur J Cardiothorac Surg 2018;53:862-70. [Crossref] [PubMed]
- Hernandez JM, Dimou F, Weber J, et al. Defining the learning curve for robotic-assisted esophagogastrectomy. J Gastrointest Surg 2013;17:1346-51. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Learning curve for Robot-assisted minimally invasive thoracoscopic esophagectomy: results from 312 cases. Ann Thorac Surg 2018;106:264-71. [Crossref] [PubMed]
- Zhang X, Su Y, Yang Y, et al. Robot assisted esophagectomy for esophageal squamous cell carcinoma. J Thorac Dis 2018;10:3767-75. [Crossref] [PubMed]
- Zhang H, Chen L, Wang Z, et al. The learning curve for robotic McKeown esophagectomy in patients with esophageal cancer. Ann Thorac Surg 2018;105:1024-30. [Crossref] [PubMed]
- van Workum F, Stenstra MHBC, Berkelmans GHK, et al. Learning curve and associated morbidity of minimally invasive esophagectomy: a retrospective multicenter study. Ann Surg 2019;269:88-94. [Crossref] [PubMed]
- Mao T, Fang W, Gu Z, et al. Comparison of perioperative outcomes between open and minimally invasive esophagectomy for esophageal cancer. Thorac Cancer 2015;6:303-6. [Crossref] [PubMed]
- Ramage L, Deguara J, Davies A, et al. Gastric tube necrosis following minimally invasive oesophagectomy is a learning curve issue. Ann R Coll Surg Engl 2013;95:329-34. [Crossref] [PubMed]
- Ma S, Yan T, Liu D, et al. Minimally invasive esophagectomy in the lateral-prone position: Experience of 124 cases in a single center. Thorac Cancer 2018;9:37-43. [Crossref] [PubMed]
- Guo W, Zou YB, Ma Z, et al. One surgeon’s learning curve for video-assisted thoracoscopic esophagectomy for esophageal cancer with the patient in lateral position: how many cases are needed to reach competence? Surg Endosc 2013;27:1346-52. [Crossref] [PubMed]
- Song SY, Na KJ, Oh SG, et al. Learning curves of minimally invasive esophageal cancer surgery. Eur J Cardiothorac Surg 2009;35:689-93. [Crossref] [PubMed]
- Osugi H, Takemura M, Higashino M, et al. Learning curve of video-assisted thoracoscopic esophagectomy and extensive lymphadenectomy for squamous cell cancer of the thoracic esophagus and results. Surg Endosc 2003;17:515-9. [Crossref] [PubMed]
- Lin J, Kang M, Chen C, et al. Thoracolaparoscopy oesophagectomy and extensive two-field lymphadenectomy for oesophageal cancer: introduction and teaching of a new technique in a high-volume centre. Eur J Cardiothorac Surg 2013;43:115-21. [Crossref] [PubMed]
- Ninomiya I, Osugi H, Tomizawa N, et al. Learning of thoracoscopic radical esophagectomy: how can the learning curve be made short and flat? Dis Esophagus 2010;23:618-26. [Crossref] [PubMed]
- Fabian T, Glotzer OS, Bakhos CT. Construct validation: simulation of thoracoscopic intrathoracic anastomosis. JSLS 2015;19(2).
- Ruurda JP, Van Der Sluis PC, Van Der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: a systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]


