
National practice trends for the surgical management of lung cancer in the CMS population: an atlas of care
Introduction
Nearly 600,000 deaths occur each year in the United States from cancer, with lung cancer being the most common cause (1). Although advanced stage lung cancer still represents the majority of newly diagnosed cases, surgery is the standard of care for early-stage non-small cell lung cancer (NSCLC), defined as clinical T1-2N0 disease. In medically operable patients, this offers the greatest chance of long-term survival (2). Although traditional surgery involves the use of an open approach, over the last two decades there has been a rising interest in minimally invasive video-assisted thoracoscopic surgery (VATS). The specific use of VATS for lobectomy has been established as a safe and effective alternative to open surgery for the management of early stage lung cancer. Several studies have associated VATS with a lower operative morbidity, transfusion requirement, chest tube duration, shorter hospital stays, and improved survival compared to open surgery (3-8). Additionally, similar long-term oncologic results between an open technique and VATS have been demonstrated (9-15).
Given that VATS can lead to improved short-term and similar long-term outcomes, the adoption of these techniques across the country has the potential to drastically impact the costs of healthcare. However, the national adoption of VATS is unknown, and generalized implementation has been challenged by debate of the regionalization of thoracic surgery to high-volume centers and the resultant financial and ethical implications related to this practice (16-18).
Currently, there is a paucity of population data regarding the surgical practice trends for the management of lung cancer in the United States, particularly in those age 65 or older. This gap represents a challenge for generating national policy, practice recommendations/guidelines, and training certifications/credentialing. Consequently, the aims of this study were to: (I) characterize open versus VATS surgical practice trends for the management of lung cancer in the United States, and (II) describe if particular regions of the country utilize minimally invasive surgery more frequently.
Methods
The Dartmouth Atlas
The Dartmouth Atlas is a statistical instrument that uses Medicare claims data to analyze and compare variables in the health care system across regions of the United States. These datasets originate from The Centers of Medicare/Medicaid (CMS) and are processed twice per year within national, regional, and local markets. A significant limitation of the Dartmouth Atlas is that the data provided is in an aggregated form for specific conditions and designated time periods (19).
The Denominator file contains 100% population beneficiary demographic and enrollment information, which is available from years 2001 to 2009. The Master Beneficiary Summary File (MBSF) is available from years 2009 forward (through 2014 at the time of this study). These two data sources are utilized during the rate generator request to establish the population of interest (19).
Data source
An exclusive national sample from the physician and supplier and denominator files from the Centers for Medicare and Medicaid Services in the years of 2006 and 2014 was utilized to identify all lung cancer diagnoses made in Medicare beneficiaries during those two years. The physician and supplier file contains all claims submitted by physicians for performance of procedures under the Medicare Part B program, including Current Procedural Terminology (CPT) codes, International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes, date of procedure, and age, sex, and race/ethnicity of the beneficiary undergoing the procedure (20-22). The denominator file contains information about eligibility by year for the Medicare Part B program and information about age, sex, and race/ethnicity of eligible beneficiaries. Our exclusion criteria included age younger than 65 years or older than 99 years and unknown race/ethnicity. This analysis was performed through an Institutional Review Board approval of the Dartmouth Atlas of Healthcare.
Hospital utilization
Inpatient hospital utilization is measured using the 100% MedPar file available for all years (currently 2001 to 2014). Each record represents a beneficiaries’ stay in an inpatient hospital and may represent more than one claim. This depends on the length of stay and the amount of services received. The MedPar file contains diagnosis codes (ICD-9 dx) and procedures codes (ICD-9 sx and DRGs) to identify the beneficiaries’ diagnosis and provided treatment (20-22).
Physician visits
Physician visits are measured using the Part B carrier file. This is available at 20% in 2002, 40% from years 2003 through 2005, and 100% thereafter (through 2014). Because it was the first year of complete data, we chose 2006 as our initial time point. Each of these records represents an individual contact from a beneficiary and a medical or associate provider. In addition, claims for labs, ambulance services, and ambulatory service centers are included in this file. For each date of service, the Part B Carrier file records include ICD-9 diagnosis codes and current CPT codes to identify the beneficiaries’ diagnosed condition and type of treatment provided (20-22).
Study population
Lung cancer diagnosed beneficiaries were identified from claim ICD-9 codes: (trachea 162.0, main bronchus 162.2, upper lobe 162.3, middle lobe 162.4, lower lobe 162.5, other parts of bronchus 162.8, and unspecified 162.9). This group of patients represented all lung cancer diagnoses in Medicare beneficiaries during each study year. Patients were then divided into those with no lung resection, those with a VATS surgical thoracoscopy with single lobectomy 32663, surgical thoracoscopy with therapeutic wedge resection 32666, surgical thoracoscopy with therapeutic additional wedge resection 32667, surgical thoracoscopy with diagnostic wedge resection followed by anatomical lung resection 32668, thoracoscopy segmentectomy 32669, thoracoscopy bilobectomy 32670, thoracoscopy pneumonectomy 32671, and those undergoing open resection (OR), as defined by thoracotomy with therapeutic wedge resection 32505, thoracotomy with additional therapeutic wedge resection 32506, thoracotomy with diagnostic wedge resection followed by anatomic lung resection 32507, thoracotomy with diagnostic biopsies of lung nodule/mass 32608, removal of lung 32440, removal of lung with segment of trachea 32442, removal of lung extrapleural 32445, single lobectomy 32480, bilobectomy 32482, removal of lung segmentectomy 32484, removal of lung with circumferential resection of segment of bronchus 32486, and removal of lung with all remaining lung follow previous removal of a portion of lung 32488. We did not include the modifier for robotic surgery (S2900), as this code was only established in July of 2005 and represents an additional procedural code to the main procedure.
Study endpoints
The primary endpoint of the analysis was the rate of VATS versus OR among patients undergoing surgical management of lung cancer during the study period. The secondary endpoint was to determine the geographic variation in use of VATS versus OR.
Statistical analysis
Rates for each of the procedures were calculated in the years of 2006 and 2014. The numerator for calculating the crude rate consisted of the number of VATS or OR procedures in each year selected; the denominator consisted of the number of beneficiaries in the 100% Medicare Part B program sample eligible. The indirect method of standardization was used to adjust for changes in age, sex, and race/ethnicity. All rates in the analysis were expressed as the number of claims per 1,000 Medicare beneficiaries and then converted to percentages.
In order to examine variation across the US in types of procedure performed, we calculated the rates of VATS and OR within each Hospital Referral Region (HRR). HRRs are defined as specific regions that represent healthcare markets with associated tertiary medical care and surgical procedures that have been validated to study geographic variation in healthcare delivery (23-28). There are currently 306 HRRs in the US with each HRR containing a minimum population size of 120,000 and at least one hospital that performs major cardiothoracic procedures. After defining crude rates of VATS and OR within each HRR for each of the years in our analysis, we indirectly adjusted each rate for differences in age, sex, and race across regions as described previously (23,28). A Student’s t-test was utilized to compare rates between regions and years. Significance across years was evaluated using nonparametric tests. Probability values <0.05 were considered significant. In addition, we created heat maps that were normalized using the concentration of events per HRR by State. All analysis was performed using SAS (SAS Institute, Cary, NC, USA), and STATA 10 (College Station, TX, USA).
Results
Incidence of lung cancer and trends of surgical management
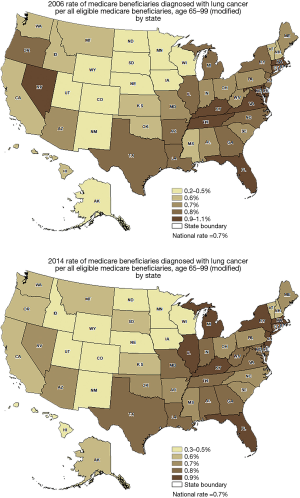
In 2006, a total of 24,368,333 Medicare beneficiaries were screened and a diagnosis of lung cancer was present in 167,418 patients (0.7%). In 2014, a total of 23,921,059 Medicare beneficiaries were screened and a diagnosis of lung cancer was present in 167,506 patients (0.7%). There was no statistical difference in the incidence of lung cancer between the years 2006 and 2014 in Medicare beneficiaries with a diagnosis of lung cancer (P=0.7) (Figure 1). Overall, the rates of lung cancer did not change appreciably during this time by state. For example, rates of lung cancer prevalence were 6.2 per 100,000 in California in 2006 and 5.6 per 100,000 in 2014 (P=0.6). Figure 2 demonstrates a set of heat maps from the years 2006 and 2014, depicting the geographic variation in the rate of a lung cancer diagnosis by state across the United States.
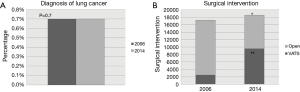
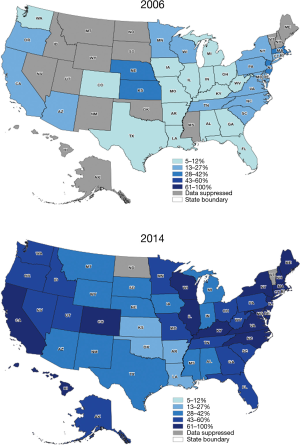
In 2006, 17,249 (10.3%) patients underwent surgery as a treatment for lung cancer, compared to 18,603 (11.1%) in 2014 (P=0.01). A VATS approach was performed in 2,512 patients (15%) during 2006 and 9,578 patients (54%) during 2014. Nationally, the rate of VATS increased during this time period by 39% (Figure 1). The rate difference over time was noted to be statistically significant (P=0.001). Figure 3 depicts the rate of VATS by state in a set of maps for the years of 2006 and 2014.
Trends in surgical management by geographic region
In 2006, California, New York, and New Jersey performed the highest total number of VATS procedures. By 2014, New York, Florida, and California performed the highest total number of VATS procedures. Table 1 lists the ten states with the most VATS interventions, both in 2006 and 2014. Overall, there was a 2-fold variation between states with Florida, New York and California having the highest total number of VATS procedures in 2014. The lowest utilization of VATS was in Colorado, Iowa, and Arkansas in 2006 compared with Utah, Alaska, and South Dakota in 2014. Interestingly, the highest percentage of VATS interventions in 2006 across the US was in New Jersey (28%), Maryland (26%), and New York (26%). In 2014, the highest percentages of VATS in states performing at least 100 surgical resections per year were in New York (70%), Massachusetts (70%), and Virginia (63%).
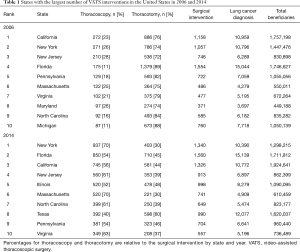
Full table
Discussion
VATS lobectomy has been associated with excellent clinical outcomes having a 30-day mortality rate of 1.9%, and a total 90-day mortality rate of 2.5% (29). In addition, a recently published study evaluating the long-term survival (5 years) of clinical stage I NSCLC based on surgical approach found similar results between minimally invasive options and thoracotomy, and the specific use of VATS was associated with a shorter length of stay (30). Furthermore, in a recent review of cost analyses, Menna et al. concluded that significant cost savings reported with VATS compared to thoracotomy for lung cancer surgery were likely related to hospital savings associated with better outcomes, particularly when an experienced surgeon performs the surgery (31).
Despite the above benefits of VATS in lung cancer surgery, our data suggest that there is clear variation in the dissemination of these techniques across the United States. Though patients were five times more likely to receive VATS procedures in 2014 as compared to 2006, significant regional variation exists in the degree to which these techniques are utilized. Some regions of the country have aggressively adopted minimally invasive surgery, while others are using it less commonly. We found the highest number of VATS cases in New York, Florida, and California in 2014. The three states with the highest percentage of VATS utilization in 2014 were New York, Massachusetts and Virginia.
Substantial geographic variation in the surgical management of NSCLC is not unique to the United States. After reviewing the use of curative surgical resection and 1-yr mortality in patients with NSCLC in England, significant geographic variation in technique was also demonstrated, even after adjustment for age, gender, and socioeconomic status (32). The authors hypothesized that these variations are the result of disparities in referral wait times, stage at diagnosis, access to non-surgical treatments, and level of involvement of thoracic surgeons. In the United States, geographic variation in the use of adjuvant therapies following resected NSCLC has also been identified (33). While there is an overall paucity of contemporary data evaluating the geographic utilization of surgery overall for NSCLC across the United States, we observed that in the CMS population, between HRRs, there was more than a 2-fold difference in the use of VATS. This suggests that there are geographical, or possible population-based, influences in the adoption of this technology and this has the potential for significant impact on surgical training and treatment availability in these regions.
To address these variations, some have advocated for the use of VATS as the standard of care for operable lung cancer with routine referral to high-volume centers and multidisciplinary treatment teams, particularly for locally advanced disease. The regionalization of thoracic surgery is a complex topic, with recent reviews questioning the actual association between volume and quality (34). In addition, surgical databases measuring quality often do not reflect patient and family satisfaction, quality of life after procedures, and the potential financial and emotional difficulties that travelling significant distances to a regional center may cause. Our data demonstrate that significant variations in the utilization of VATS techniques exist across geographic regions in the United States in the treatment of NSCLC. Now that these differences have been identified, further studies are required to help clarify the underlying issues related to the adoption of VATS in areas with low utilization.
Study limitations
Our analysis has several limitations related to the use of an administrative database. First, patients in this analysis are Medicare recipients, and thus the results must be understood in the context of patients being ≥65 years of age. Second, patients were identified in our analysis based on Part B CPT codes for thoracoscopic procedures or thoracotomy, and miscoding of procedures may have occurred. It is not possible to determine whether the use of thoracoscopy increased secondary to a higher number of diagnostic resections rather than therapeutic resections, although the intent of the surgical procedure may be inferred based on the CPT codes. Lastly, this analysis does not contain demographic information on the study population or data on in-hospital morbidity or long-term survival, as it is aggregated HRR level data. There is no available information regarding cancer stage or the type of surgeon performing the procedures. Despite these limitations, we feel the study adds meaningful data on the contemporary adoption of VATS in the treatment of NSCLC across the United States, and demonstrates the variation in types of surgical interventions delivered between different regions of the country.
Conclusions
While the prevalence of lung cancer in the United States was unchanged from 2006 to 2014 in the CMS population, surgical management of NSCLC has increased over time, in this population. A change in surgical practice patterns was evident, with a significant increase in the use of VATS techniques in more than 50% of cases after this eight-year period. There is need for future studies focused on investigating variables that affect the use or availability of VATS across regions of the country, and in the non-CMS population.
Acknowledgements
None.
Footnote
Conflict of Interest: Podium presentation: Academic Surgical Congress 2018. January 30th to February 1st in Jacksonville, FL.
Ethical Statement: This analysis was performed through an Institutional Review Board approval of the Dartmouth Atlas of Healthcare.
References
- Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013.
- Nur U, Quaresma M, De Stavola B, et al. Inequalities in non-small cell lung cancer treatment and mortality. J Epidemiol Community Health 2015;69:985-92. [Crossref] [PubMed]
- Jones GC, Kehrer JD, Kahn J, et al. Primary treatment options for high-risk/medically inoperable early stage NSCLC patients. Clin Lung Cancer 2015;16:413-30. [Crossref] [PubMed]
- Yang SM, Hsu HH, Chen JS. Recent advances in surgical management of early lung cancer. J Formos Med Assoc 2017;116:917-23. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Phillips JD, Merkow RP, Sherman KL, et al. Factors affecting selection of operative approach and subsequent short-term outcomes after anatomic resection for lung cancer. J Am Coll Surg 2012;215:206-15. [Crossref] [PubMed]
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28, dicussion 28-29.e1.
- Smith CB, Wolf A, Mhango G, et al. Impact of Surgeon Volume on Outcomes of Older Stage I Lung Cancer Patients Treated via Video-assisted Thoracoscopic Surgery. Semin Thorac Cardiovasc Surg 2017;29:223-30. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg 2008;135:642-7. [Crossref] [PubMed]
- Swanson SJ, Herndon JE, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802—a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Schrag D, Earle C, Xu F, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst 2006;98:163-71. [Crossref] [PubMed]
- Kozower BD, Stukenborg GJ. Volume-outcome relationships in thoracic surgery. Thorac Surg Clin 2017;27:251-6. [Crossref] [PubMed]
- Schrag D, Panageas KS, Riedel E, et al. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. J Surg Oncol 2003;83:68-78. [Crossref] [PubMed]
- Vannucci F, Gonzalez-Rivas D. Is VATS lobectomy standard of care for operable non-small cell lung cancer? Lung Cancer 2016;100:114-9. [Crossref] [PubMed]
- The Dartmouth Atlas of Healthcare. Available online: www.dartmouthatlas.org
- Dartmouth Synergy Program: The Atlas Rate Generator. Available online: https://ctpr.dartmouth.edu/rate_requests/
- American Medical Association Web site. CPT (Current Procedural Terminology). Available online: https://www.ama-assn.org/amaone/cpt-current-procedural-terminology, accessed Jan 1, 2019.
- World Health Organization, International Classification of Diseases, Ninth Revision (ICD9). Geneva, Switzerland World Health Organization, 1977.
- Goodney PP, Lucas FL, Travis LL, et al. Changes in the use of carotid revascularization among the medicare population. Arch Surg 2008;143:170-3. [Crossref] [PubMed]
- Iribarne A, Goodney PP, Flores AM, et al. National trends and geographic variation in bilateral internal mammary artery use in the United States. Ann Thorac Surg 2017;104:1902-7. [Crossref] [PubMed]
- Available online: : Dartmouth Atlas of Healthcare. Accessed June 8, 2016.www.dartmouthatlas.org
- Huber TS, Seeger JM. Dartmouth Atlas of Vascular Health Care review: impact of hospital volume, surgeon volume, and training on outcome. J Vasc Surg 2001;34:751-6. [Crossref] [PubMed]
- Newman L. New Dartmouth Atlas: improving US cardiac care? Lancet 2000;356:660. [Crossref] [PubMed]
- Cooper MM. The Dartmouth Atlas of Health Care: what is it telling us? Health Syst Rev 1996;29:44-5, 47. [PubMed]
- Brunelli A, Dinesh P, Woodcock-Shaw J, et al. Ninety-Day Mortality After Video-Assisted Thoracoscopic Lobectomy: Incidence and Risk Factors. Ann Thorac Surg 2017;104:1020-6. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term survival based on the surgical approach to lobectomy for clinical stage I nonsmall cell lung cancer: comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Menna C, Ibrahim M, Rendina EA, et al. Cost/efficacy evaluation of the technologies applied to video-assisted thoracoscopic surgery lobectomy. J Vis Surg 2017;3:152. [Crossref] [PubMed]
- Tataru D, Spencer K, Bates A, et al. Variation in geographical treatment intensity affects survival of non-small cell lung cancer patients in England. Cancer Epidemiol 2018;57:13-23. [Crossref] [PubMed]
- Schroeder MC, Tien YY, Wright K, et al. Geographic variation in the use of adjuvant therapy among elderly patients with resected non-small cell lung cancer. Lung Cancer 2016;95:28-34. [Crossref] [PubMed]
- Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med 2001;345:181-8. [Crossref] [PubMed]


