
Comparison of lymph node dissection and lymph node sampling for non-small cell lung cancers by video-assisted thoracoscopic surgery
Introduction
Lung cancer is the leading cause of cancer-related deaths and nearly 1.6 million deaths are expected worldwide annually (1). Lobectomy with lymph node sampling (LNS) or lymph node dissection (LND) is generally accepted as the standard procedure for medically operable patients with NSCLC. There have been increasing evidences showing that compared to open thoracotomy, lobectomy via video-assisted thoracoscopic surgery (VATS) is associated with less postoperative pain, shorter chest drain duration and hospital stay (2,3). The long-term efficacy of lobectomy for clinical stage I lung cancer performed by VATS is not inferior to thoracotomy (4). It has now become recommended approach in clinical guidelines (5), accounting for more than 15% of lobectomies performed in the United States (6) to over 50% in large volume centers in China (7). However, one critical concern about minimally invasive lung cancer surgery is whether appropriate LND could be achieved by VATS (8,9). Although several randomized trials have compared the surgical and oncological outcomes between LNS and LND (10-14), most surgical approaches in those studies were open thoracotomy. It is still controversial whether LND by VATS is safe and feasible. We hereby performed a retrospective study to compare the efficacy of LND and LNS in VATS lobectomy patients with resectable NSCLC and their perioperative results using propensity-score matching.
Methods
Patients with primary lung cancers referred for lobectomy and LND or sampling by VATS at our unit between January 2012 and December 2016 were retrospectively selected from a prospectively maintained database. All patients were diagnosed with clinical stage I–IIIa diseases before operation. Preoperative workup included computed tomography (CT) of the chest, neck and abdominal ultrasonography for all the patients in this study. Patients with solid pulmonary nodule or mixed ground glass opacity (GGO) with more than 50% solid component also had magnetic resonance imaging (MRI) of brain and positron emission tomography (PET) scan. Fibrous bronchoscopy was not indicated in lesions located in the outer 1/3 of the pulmonary parenchyma. Mediastinoscopic biopsy or endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was only performed for patients with suspected positive bulky mediastinal lymph nodes basing on PET scan. Histological confirmed N2 diseases before operation were excluded in this study. So the clinical N2 diseases were diagnosed only by image studies. All patients received lobectomy by VATS with tri-portal approach, with systemic LND or sampling, with patients receiving wedge resection, segmentectomy, bilobectomy and pneumonectomy excluded from the study. The extent and definition of LND and sampling was explained and performed according to the European Society of Thoracic Surgeons (ESTS) guideline (15). LND was indicated in patients with solid pulmonary nodule and mGGO with more than 50% solid component. A skeletonized LND removing all mediastinal lymph nodes together with surrounding fatty tissue was carried out according to the same standard in open surgery (13). Mediastinal LNS was reserved for patients with pure GGO or mixed GGO with less than 50% solid component or those considered of high surgical risks because of compromised cardiopulmonary functions. But hilar and intrapulmonary lymph nodes were all excised as the minimum requirement. All the procedures were performed by surgeons from a single team.
Comparisons between proportions were made by Pearson’s χ2 test or Fisher’s exact test. Continuous variables were described as means and standard deviations or medians and range and Student t-test or Mann-Whitney test was used for comparison between two groups. Potential risk factors with a P value less than 0.1 were entered into multivariate analysis by logistic regression to identify independent risk factors for mediastinal lymph node metastasis. As the baseline characteristics in the two groups were not balanced, a propensity-score matched analysis was performed to compare perioperative results after LND or LNS. Patients were matched at a ratio of 1:1 with caliper distance limited to 20%. All statistical analyses were performed using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). P values were 2-sided and those less than 0.05 were accepted as statistically significant.
Results
Among 773 VATS lobectomy patients included in this study, 494 (63.9%) patients received LND and 279 (36.1%) patients had LNS. Patient demographics and tumor characteristics are summarized in Table S1. There were more male patients, more lower lobe and higher pathological T/N stage tumors, more squamous histology but less adenocarcinomas in the LND group than in the LNS group. Rate of adenocarcinomas with micropapillary or solid predominant component was also significantly higher in the LND group than in the LNS group. There was no significant difference in age or co-morbidity between two groups.
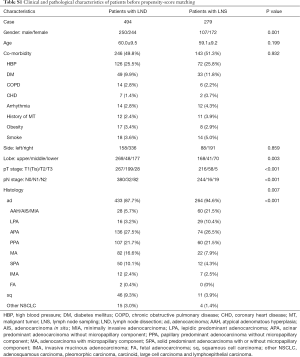
Full table
Perioperative results are shown in Table 1. More lymph nodes and number of stations of nodes were harvested in the LND group than in the LNS group. After surgery, only one patient died of pulmonary embolism in the LND group. Comparing to the LNS group, patients in the LND group had significantly longer operation time, higher amount of postoperative drainage, longer postoperative hospital stay and higher morbidity rate.
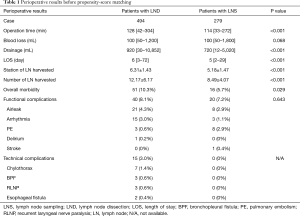
Full table
Propensity-score matching resulted in a final cohort of 558 patients (279 LND and 279 LNS) for further analysis of peri-operative results (Table 2). No statistics significance was observed in clinicopathological characteristics between the two groups except for the pN stage (Table 3). Number of lymph nodes harvested and number of stations of nodes dissected were still significantly higher in the LND group than in the LNS after matching. Patients in the LND group still had significantly longer operative time (but only 14 minutes), more postoperative drainage (200 mL), and longer postoperative hospital stay (1 day only) than patients in the LNS group. Statistical significance in post-operative morbidity was no longer observed between the two groups (8.6% after LND vs. 4.7% after LNS). However, technical complications such as bronchopleural fistula, esophageal fistula, chylothorax, and recurrent laryngeal nerve paralysis were seen only in the LND group.
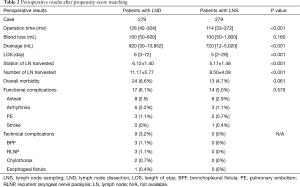
Full table

Full table
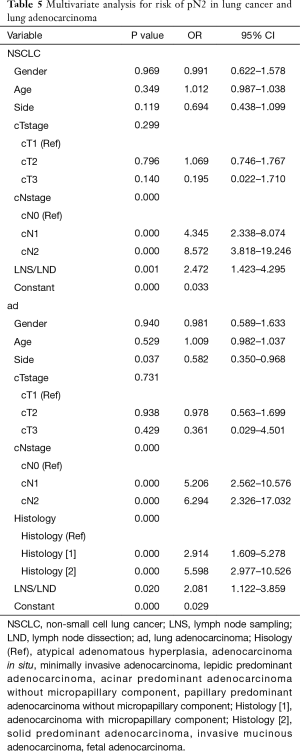
Among all 773 patients enrolled in this study, 101 patients had pN2 lung cancers. Clinicopathological characteristics of patients with or without pN2 disease are shown in Table 4. Significantly more male, more left sided and higher clinical N stage tumors were observed in patients with pN2 disease. Multivariate analysis suggested that clinical N stages higher than cN0 category and LND were independent risk factors for detecting pN2 diseases in all lung cancers (Table 5).
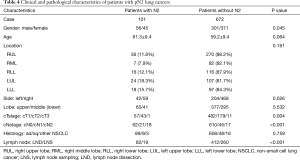
Full table
Full table
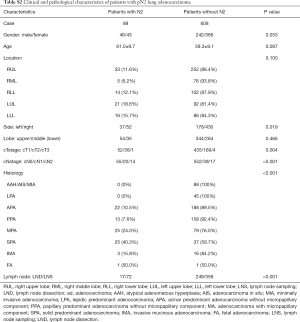
Since most of the tumors in this series were adenocarcinomas, clinicopathological characteristics of 697 patients with lung adenocarcinoma were then studied separately. Significantly more male, more left sided and higher clinical N stage tumors, and more micropapillary or solid predominant or mucinous lesions were observed in pN2 lung adenocarcinomas (Table S2). Upon multivariate analysis, clinical N stages higher than cN0 category, solid or micropapillary component or mucinous adenocarcinoma or fetal adenocarcinoma, and LND were independently related to finding pN2 stage in adenocarcinomas (Table 5). However, due to the low rate of mucinous and fetal subtype in all adenocarcinomas, it is safe to only conclude solid or micropapillary component as an independent risk factor for detecting pN2 diseases in adenocarcinomas.
Full table
Discussion
In this study we carried out a propensity-score matched analysis to compare perioperative results between LND and LNS by VATS. Although LND was associated with statistically longer operation time, more postoperative drainage, and longer postoperative stay than LNS, the differences were of limited clinical significance. And difference in overall morbidity rates was only of borderline significance after LND and LNS. However, technical complications such as bronchopleural fistula, esophageal fistula, chylothorax, and recurrent laryngeal nerve paralysis were seen only in the LND group. On the other hand, multivariate analysis revealed higher clinical N stage category as an independent predictor for pN2 disease in all histologies, and micropapillary and solid predominant subtypes were found to be independent risk factors for pN2 involvement in lung adenocarcinomas. In both overall analysis or in adenocarcinomas specifically, LND turned out to be an independent risk factor for detecting pN2 nodal status.
One major concern for LND is whether it would increase surgical risks. It is still unclear whether LND by VATS is as safe as LNS. In the meantime, it is generally accepted that LND is indispensible for accurate pathologic staging. Previous study also proved mediastinal LND might have survival advantage when comparing with LNS in patients with resectable NSCLC (16). With minimally invasive surgery increasingly often used in management of lung cancers, concern has also been raised on whether similarly adequate nodal dissection could be accomplished under VATS as in open thoracotomy (17-19), making it necessary to evaluate the safety and efficacy of LND in VATS lobectomy.
In the current study, all patients received VATS lobectomy. And propensity-score matching was used in analysis of perioperative outcomes to reduce the potential influence of confounding biases to the greatest extent. Mean operation time was prolonged for merely 14 minutes, which was similar to the result of the ACOSOG Z0030 trial (10). Average postoperative drainage amount increased by only 200 mL in total, and hospital stay was prolonged for only 1 day after operation. These results suggest that LND under VATS has limited additional impact on the operative process or postoperative course, as compared with LNS only.
In our study only one patient died of pulmonary embolism after surgery, which was unrelated to extent of nodal dissection. Overall morbidity for LND and LNS was 10.3% and 5.7% in all patients, much lower than in the ACOSOG Z0030 trial (10). Several reasons may account for this result. First of all, only lobectomy cases were included in our study, while there were 42 pneumonectomies and 42 bilobectomies in the ACOSOG Z0030 trial. Second, surgical approach in our patients was solely by VATS, while 90% patients in the ACOSOG Z0030 trial received open thoracotomy. Lastly, the two studies were carried out in different time period. Continuing improvement in operative techniques and postoperative management might also have contributed to decreased morbidity.
Although no statistics significance was observed in overall morbidity between the two matched groups, certain technical complications such as bronchopleural fistula, esophageal fistula, chylothorax, and recurrent laryngeal nerve paralysis were noticed only in the LND group. Satoh proposed that decreased blood supply to the bronchial stump contributed to BPF (20). In this concern, ligation of bronchial artery during skeletonized LND in our patients might have affected the blood supply to the bronchus and increased the risk of BPF. Two patients developed small esophageal fistula after LND. Since there was no tumor invasion into the esophagus in these two cases, most probably this was caused by thermal injury from harmonic scalpel during LND in the subcarinal area. Incidence of chylothorax after LND was reported to be 2.1%-2.4%, with a higher incidence on the right side than on the left side (21,22). In our study, chylothorax happened in 1.4% cases and all of them were on the right side. It is likely due to damage of the lymphatic branches in the right upper mediastinum during dissection of 2R and 4R lymph nodes. Vocal cord paralysis was previously reported to be 3.7–31% in patients who underwent thoracic surgery, with a higher incidence on the left side (23-25). All cases of recurrent laryngeal nerve paralysis happened on the left side in our study. This is likely related to the location of the recurrent nerves as it is close by lymph node stations 4 and 5 on the left side but posterior to the vagus nerve on the right side where nodal dissection is mainly carried out anteriorly. These technical complications may be related to the skeletonized mediastinal dissection which has been our standard procedure. Care should be taken in the future to avoid the surgical risks associated with these technical problems.
In our study in VATS lobectomy patients, significantly more number (12.2 vs. 8.5, P<0.001) and stations (6.3 vs. 5.2, P<0.001) of lymph nodes were resected in the LND group than in the LNS group. Upon multivariate risk analyses for pN2 disease, LND turned out to be an independent predictor both in all lung cancer histologies and in adenocarcinomas alone. The results indicated that systemic nodal dissection may help increase the accuracy of nodal staging in minimally invasive lung cancer surgery. Although only 4.5% patients were staged as cN2 before surgery in this study, 16.8% and 6.8% of them turned out to have N2 disease after LND and LNS (P<0.001). Our result was different from the ACOSOG Z0030 trial which showed merely 4% upstaging after LND (10). But in the ACOSOG Z0030 trial only patients with T1-2 tumors and non-hilar N1 were included, mediastinoscopy was used more often, and randomization was after negative mediastinal nodal sampling. The results are therefore not generalizable to patients staged radiographically or those with higher T stage tumors (10). It is interesting to notice that although clinical T staging was associated with higher rate of pN2 both in all lung cancers and in adenocarcinomas alone, it was not revealed as an independent risk factor for detecting pN2 disease in multivariate analyses. Previous study also showed the size of lesions was associated with risk of nodal involvement and it was the rate of lymph node metastasis that delineate the survival difference among different size categories in lung cancers (26). Preoperative N staging in our patients depended mostly on imaging studies, and PET scan or mediastinoscopy was not routinely used before operation, which explained for the high LND rate (63.9%) in this series (27). In addition to clinical N staging, LND was also an independent predictive factor for revealing pN2 disease. The findings of our study indicate that adequate LND should still be the surgical standard in radiographically staged patients and can be achieved by VATS as well as in open thoracotomy.
In the current series, no patient with AIS, MIA, or lepidic dominant adenocarcinomas were found to have pN2 disease, which was in accordance with the existing literatures (28-30). N2 involvement was found only in 6.6% and 10.5% of patients with papillary and acinar adenocarcinomas. But in micropapillary or solid dominant adenocarcinomas rate of mediastinal nodal metastasis was as high as 24.0% and 40.3%. Upon multivariate analysis in adenocarcinomas, histology subtypes was also revealed as an independent risk factor for N2 disease. Considering that patients with cN1 and cN2 tumors were associated with significantly increased risk of having pN2 metastasis than patients with cN0 tumors in both multivariate analysis, it seems that LNS may be acceptable for patients with cN0 tumors, and those with adenocarcinomas without micropapillary or solid component could be exempted from systemic LND and its associated surgical risks. But for patients with tumors in higher clinical N stage categories or more invasive histology, LND is still indispensible to ensure accurate tumor staging and completeness of resection.
There were certain limitations in this study, given its retrospective nature. Selection biases in treatment allocation were inevitable even though propensity-score matching was used. More patients with solid pulmonary nodule and mixed GGO with solid component more than 50% had LND than LNS, but this was less likely to be related to surgical morbidity. And upon multivariate analysis, LND was still found to be an independent predictive factor for detecting pN2 diseases. With minimally invasive approaches increasingly accepted in lung cancer surgery, a prospective study comparing LND and LNS under VATS would be helpful to further elucidate the safety and value of LND.
In conclusion, LND by VATS has acceptable perioperative results comparing with LNS. And systemic nodal dissection under VATS can help improve accuracy of staging by detecting more N2 disease as it does in open surgery. Although its long-term influence on patient survival still awaits further follow-up, LND should be an indispensable part of both minimally invasive and open surgery for NSCLC, especially those in higher clinical N categories and those with more invasive histologies. Since LND could be carried out in equal safety and efficacy under VATS as in open surgery, it should be taken as an integrated part of minimally invasive surgery for the treatment of resectable NSCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of the hospital (No. ks11318) and written informed consent was obtained from all patients.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Furnary AP, et al. Minimally Invasive Lung Cancer Surgery Performed by Thoracic Surgeons as Effective as Thoracotomy. J Clin Oncol 2018;36:2378-85. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer, Version 3. 2017. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 16 Nov 2016.
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Sihoe ADL, Han B, Yang TY, et al. The Advent of Ultra-high Volume Thoracic Surgical Centers in Shanghai. World J Surg 2017;41:2758-68. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53. [Crossref] [PubMed]
- Licht PB, Jorgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9. [Crossref] [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9. [Crossref] [PubMed]
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44. [Crossref] [PubMed]
- Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290-4; discussion 294-5. [Crossref] [PubMed]
- Zhang J, Mao T, Gu Z, et al. Comparison of complete and minimal mediastinal lymph node dissection for non-small cell lung cancer: Results of a prospective randomized trial. Thorac Cancer 2013;4:416-21. [Crossref] [PubMed]
- Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Wright G, Manser RL, Byrnes G, et al. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax 2006;61:597-603. [Crossref] [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [Crossref] [PubMed]
- Scott WJ, Matteotti RS, Egleston BL, et al. A comparison of perioperative outcomes of video-assisted thoracic surgical (VATS) lobectomy with open thoracotomy and lobectomy: results of an analysis using propensity score based weighting. Ann Surg Innov Res 2010;4:1. [Crossref] [PubMed]
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010; 89:1730-5; discussion 1736.
- Satoh Y, Okumura S, Nakagawa K, et al. Postoperative ischemic change in bronchial stumps after primary lung cancer resection. Eur J Cardiothorac Surg 2006;30:172-6. [Crossref] [PubMed]
- Shimizu K, Yoshida J, Nishimura M, et al. Treatment strategy for chylothorax after pulmonary resection and lymph node dissection for lung cancer. J Thorac Cardiovasc Surg 2002;124:499-502. [Crossref] [PubMed]
- Cho HJ, Kim DK, Lee GD, et al. Chylothorax complicating pulmonary resection for lung cancer: effective management and pleurodesis. Ann Thorac Surg 2014;97:408-13. [Crossref] [PubMed]
- Zumtobel M, End A, Bigenzahn W, et al. Reduced quality of life in patients with unilateral vocal cord paralysis after thoracic surgery. Chirurg 2006;77:518-22. [Crossref] [PubMed]
- Schneider B, Schickinger-Fischer B, Zumtobel M, et al. Concept for diagnosis and therapy of unilateral recurrent laryngeal nerve paralysis following thoracic surgery. Thorac Cardiovasc Surg 2003;51:327-31. [Crossref] [PubMed]
- Filaire M, Mom T, Laurent S, et al. Vocal cord dysfunction after left lung resection for cancer. Eur J Cardiothorac Surg 2001;20:705-11. [Crossref] [PubMed]
- Sagawa M, Saito Y, Takahashi S, et al. Clinical and prognostic assessment of patients with resected small peripheral lung cancer lesions. Cancer 1990;66:2653-7. [Crossref] [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6. [Crossref] [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg 2014;98:453-8. [Crossref] [PubMed]
- Yu Y, Jian H, Shen L, et al. Lymph node involvement influenced by lungadenocarcinoma subtypes in tumor size ≤3 cm disease: A study of 2268 cases. Eur J Surg Oncol 2016;42:1714-9. [Crossref] [PubMed]


