Pulmonary sparganosis mansoni: a case report from a non-endemic region
Introduction
Sparganosis mansoni is an infectious disease caused by the plerocercoid larvae (spargana) of various diphyllobothroid tapeworms belonging to the genus Spirometra and occurs in humans and animals (1). Sparganosis mansoni has been reported sporadically worldwide, but its prevalence is higher in several Asian countries, including South Korea, Japan, Thailand, and China (2,3). The cases of eye sparganosis and subcutaneous sparganosis have been reported previously, but very few cases of pulmonary sparganosis mansoni have been reported thus far (4). Here, we presented a case of pulmonary sparganosis mansoni in Shanghai, China.
Case report
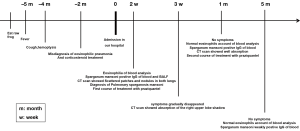
The case was of a 32-year-old Chinese woman. She had a steady job in an office and never travelled overseas. In May 2010, the patient complained of fever lasting for 1 month, with a maximum body temperature 38.2 °C. In June, she reported intermittent bloody phlegm occurring approximately 2-3 times per day. In October 2010, she was admitted to a tertiary hospital. After examinations, she was diagnosed as eosinophilic pneumonia; she was subsequently treated with 40 mg corticosteroid, and the dose was tapered by 5 mg every week. Despite corticosteroid treatment for 2 months, her chest computed tomography (CT) showed recurrent lesions, and she still complained of persistent cough and expectoration. She was therefore transferred to our hospital for a detailed examination. On thoroughly history taking, we found that the patient had a history of eating raw seafood and a preference for raw frogs and bullfrogs. On admission to our hospital, her vital signs were stable. A few inspiratory crackles were noted in the right lower lung lobe. Initial blood analysis showed elevated cells counts: peripheral white blood cell count, 10.5×109/L and eosinophils count, 0.69×109/L. Administration of oral prednisone was discontinued, and she was started on intravenous cefuroxime (1.5 g bid) and clindamycin (0.6 g bid) for 9 days. Chest CT (10 days after admission) revealed scattered patches and nodules in both lungs (Figure 1A,B). Subsequently, bronchoscopy was performed, but there were no remarkable findings. The blood was tested at the Chinese Centre for Disease Control Institute of Parasitic Disease and Parasitology (CDC) to test for the presence of antibodies to any parasites. The results revealed a suspiciously positive result for antibodies against Sparganum mansoni. Re-analysis of the blood, and the bronchoalveolar lavage fluid confirmed the presence of IgG antibodies against Sparganum mansoni. The brain and other organs such as liver, kidney, heart, and spleen were also examined. Thereafter, the patient was diagnosed with pulmonary sparganosis mansoni and treated with praziquantel tablets (600 mg tid) orally for 5 days. After treatment, the symptoms gradually disappeared. Review of the chest CT (20 days after admission) revealed absorption of the right upper lobe shadow (Figure 1C,D). One month later, the patient had no cough and expectoration. Blood analysis showed a normal eosinophil account. However the blood mansoni sparganosis antibody was still positive. In comparison with the previous scan, the chest CT scan showed good absorption of the right upper lobe lesion (Figure 1E,F). She received a second treatment dose of praziquantel tablets (600 mg tid) orally for 5 days. Following-up examination conducted 5 months after the treatment showed that the blood IgG antibody against Sparganum mansoni was weakly positive, and blood eosinophils account was normal.
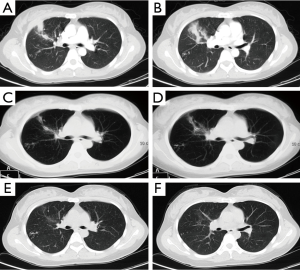
It took nearly 6 months to make a final correct diagnosis of pulomary sparganosis mansoni. After two courses of oral praziquantel and 5 months of follow-up, the patient mainly recovered (Figure 2).
Discussion
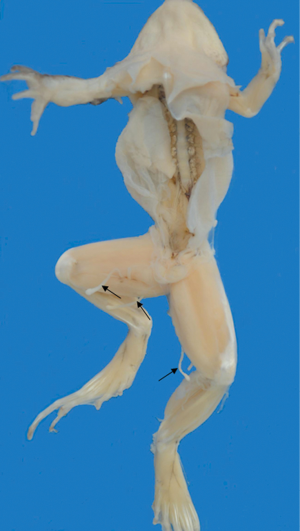
In recent years, with improvements in the standard of living and changes in dietary habits, the incidence of sparganosis mansoni and other food-borne parasitic diseases has increased in China; this is a constant concern to food safety and community health (5). Sparganosis mansoni has been noted in China, especially in Zhejiang, Fujian, Guangdong provinces in the southeast coastal region except in Shanghai. Sparganum mansoni is caused by the larvae of Spirometra mansoni. The main sources of infection are cats, frogs, dogs, and other animals, which are intermediate hosts. Humans, who are also a second-intermediate host and paratenic hosts, can be infected with spargana by drinking water contaminated with procercoid-infected copepods, eating undercooked meat of snakes or frogs infected with spargana, or using poultices of frog or snake flesh or skin on open wounds (1,6). The current patient was a woman who lived and worked in Shanghai. In our case, we found several Sparganum mansoni in a frog sample provided by the patient (Figure 3). Thus, infection probably occurred due to consumption of undercooked frogs and bullfrogs.
When humans ingest raw frogs or snakes, they also consume the plerocercoid larvae, which can cause infection. The parasites have strong migration and proliferation abilities and can usually pass through the intestinal wall and the peritoneum, finally reaching the subcutaneous tissues. The plerocercoid larvae rarely affect internal organs such as the lungs, brain, and spinal cord. According to clinical symptoms and location of the parasite, sparganosis mansoni are of 5 types: eye sparganosis, subcutaneous sparganosis, oral and maxillofacial sparganosis, brain sparganosis, and visceral sparganosis (7). Among these, visceral sparganosis is rare, compromising only 1% of all cases. Thus, pulmonary involvement is rarely reported in the literature (4). The patient reported here repeatedly presented with intermittent cough, hemoptysis and increased eosinophil count. Chest CT showed migratory patch shadows in both lungs. Thus, the diagnosis was confused with eosinophilic pneumonia due to the clinical features. Physicians should be cautious when corticosteroid therapy shows no effect on lesion absorption in chest radiology.
Considering the small number of cases of sparganosis mansoni in the literature and its confusing clinical features, the diagnostic method used for this infection should be quick and easy, with high sensitivity and specificity. The result should also be verified by using single copy of the gold standard immunoconcentration assay or immunochromatography (8). In our case, considering the patient’s increased eosinophils count and food habits, we decided to send her samples to CDC for urgent testing for parasites antibodies. A multiple-dot enzyme-linked immunosorbent assay of the serum and bronchoalveolar lavage fluid showed repeated positive results for the anti-sparganum antibodies. Unfortunately, the patient refused to undergo transbronchial lung biopsy or video-assisted thoracoscopic surgery lung biopsy. Unlike the case reported previously by Iwatani K et al. (9), there was no histopathological confirmation in this case. However, considering the epidemiology, clinical features, chest CT findings, and positive blood test results and antibodies, a final clinical diagnosis of pulmonary sparganosis mansoni was made.
Currently, more physicians recommend surgery as the best treatment for cerebral sparganosis mansoni (10). Since very few reports on pulmonary sparganosis mansoni exist in the literature, it was difficult to find information on the condition. Our patient presented with transmigrating lesions in both lungs. Since surgery was not appropriate, we treated her with two courses of oral praziquantel tablets without corticosteroid therapy. After 1 month, her peripheral blood eosinophil count returned to normal. The patient’s symptoms were significantly improved and radiological lesions resolved. Five months later, the sparganum mansoni serum antibody was weakly positive. The incidence of lung sparganosis mansoni is rare. It is not well diagnosed and often misdiagnosed as either eosinophilic pneumonia or hypersensitivity pneumonitis owing to its clinical features (Table 1). The clinical diagnosis of this disease must be made considering the epidemiology, clinical manifestations, chest imaging findings, and serological or alveolar lavage fluid analysis, relevant antibody testing,as well as patient history.
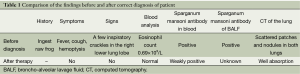
Full table
Conclusions
Pulmonary sparganosis mansoni is a rare condition. To our knowledge, this is the first case of pulmonary sparganosis mansoni reported in east China. Oral praziquantel treatment is effective, although a repeated course of therapy may be required. Epidemiological investigation is important but not necessary when pulmonary parasites are suspected in a patient with lung lesions. Patients should be advised to avoid ingestion of raw frogs or snakes, or exposing any wounds to raw frog meal in order to prevent the disease in endemic and non- endemic region.
Acknowledgements
We thank all study participants for their cooperation, technical help, and sample collection. We are grateful to all members of the Chinese Centre for Disease Control Institute of Parasitic Disease and Parasitology, for their knowledge, helpfulness, and willingness to contribute. This study was supported by National Science Foundation of China [NSFC81170003], Shanghai Elite Medical Talent Project [XYQ2011006] and Project from STCSM [12PJD004, 12JC1402300, 134119a6400].
Disclousure: The authors declare no conflict of interest.
References
- Li MW, Song HQ, Li C, et al. Sparganosis in mainland China. Int J Infect Dis 2011;15:e154-6. [PubMed]
- Shin EH, Guk SM, Kim HJ, et al. Trends in parasitic diseases in the Republic of Korea. Trends Parasitol 2008;24:143-50. [PubMed]
- Anantaphruti MT, Nawa Y, Vanvanitchai Y. Human sparganosis in Thailand: an overview. Acta Trop 2011;118:171-6. [PubMed]
- Huang F, Gong HY, Lu MH. Pulmonary sparganosis mansoni: a case report. Trop Biomed 2012;29:220-3. [PubMed]
- Coordinating Office of the National Survey on the Important Human Parasitic Diseases. A national survey on current status of the important parasitic diseases in human population. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005;23:332-40. [PubMed]
- Murata K, Abe T, Gohda M, et al. Difficulty in diagnosing a case with apparent sequel cerebral sparganosis. Surg Neurol 2007;67:409-11; discussion 412. [PubMed]
- Oh SI, Koh SH, Pyo JY, et al. Sparganosis mimicking an intramedullary tumor of the cervical cord. J Clin Neurosci 2011;18:1128-9. [PubMed]
- Chung YB, Kong Y, Yang HJ, et al. IgG antibody responses in early experimental sparganosis and IgG subclass responses in human sparganosis. Korean J Parasitol 2000;38:145-50. [PubMed]
- Iwatani K, Kubota I, Hirotsu Y, et al. Sparganum mansoni parasitic infection in the lung showing a nodule. Pathol Int 2006;56:674-7. [PubMed]
- Hong D, Xie H, Zhu M, et al. Cerebral sparganosis in mainland Chinese patients. J Clin Neurosci 2013;20:1514-9. [PubMed]