Single or dual antiplatelet therapy after transcatheter aortic valve replacement: an updated systemic review and meta-analysis
Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as an essential therapeutic strategy for patients with severe, symptomatic aortic valve stenosis, especially for those with contraindications as well as a high risk of surgical aortic valve replacement (1). Despite the high success rate of TAVR, two common complications following TAVR, stroke and bleeding, deserve more attention with the widespread application of this procedure. The PARTNER 2A and SURTAVI trials showed that, in patients with intermediate surgical risks, the incidence rates of cerebral ischemia and major bleeding within 30 days after TAVR were about 5% and 10%, respectively (2,3).
Currently, dual antiplatelet therapy (DAPT) with aspirin and clopidogrel is the most commonly used post-TAVR antiplatelet regimen in clinical studies. The 2014 AHA/ACC guidelines (4) recommended the application of DAPT for 6 months after TAVR in patients with no indication for anticoagulants (class IIb, level of the evidence C) and this recommendation was retained in the updated edition of 2017 (1). Nevertheless, the recommendation is not based on the results of large randomized trials. On the other hand, Aryal et al. and Gandhi et al. suggested that the effect of single antiplatelet therapy (SAPT) would be noninferior to DAPT and the application of SAPT was even more likely to reduce the risk of hemorrhage (5,6). However, previous meta-analyses are, for the most part, inadequate to identify the differences between two groups due to small sample sizes. Therefore, we conducted a refined meta-analysis of the recent randomized and observational studies to acquire a better understanding of the safety and efficacy of the two post-TAVR antiplatelet therapies.
Methods
This systemic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) statement recommended by the Cochrane Collaboration (7).
Search strategy and selection criteria
PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched to retrieve relevant studies published from inception to Feb 19, 2018. There is no language restriction in our search. We used a combination of MeSH/Emtree and entry terms of TAVR, platelet aggregation inhibitors, clopidogrel, aspirin, prasugrel, ticagrelor and antiplatelet as search keywords to locate relevant entries. The details of search strategy is shown in Supplementary Data. Manual search was also performed to identify additional publications from the reference lists of related reviews and meta-analyses.
Study selection
The inclusion criteria were as follows: (I) randomized controlled or observational studies, (II) patients undergoing TAVR, (III) direct comparison between SAPT and DAPT after TAVR. Studies were excluded if they were duplicates, conference abstracts or rather missing the outcomes of interest. Two of the researchers screened the electronic records and retrieved publications independently and any discrepancy was resolved through discussing and reading the full text of the article. If necessary, a final reviewer resolved the disagreement.
The safety and efficacy of the two regimens was compared for 30-day outcomes and mortality beyond 3 months. The primary outcome in the current study was 30-day death, while the secondary outcomes are 30-day stroke (major and minor), life-threatening or major bleeding, spontaneous myocardial infarction (MI), as defined by Valve Academic Research Consortium (VARC)-2 (8). The death beyond 3 months was also considered as another secondary outcome.
Data extraction and quality assessment
Two of the investigators extracted the characteristics and data from the retrieved studies independently. We used Cochrane Collaboration tool (by Review Manager 5.3) and Newcastle-Ottawa scale (9) to assess the risk of bias for randomized controlled trials (RCTs) and observational studies, respectively. The quality of evidence was assessed by the scoring system, GRADE (Grading of Recommendations Assessment, Development and Evaluation). The software, GRADEprofiler 3.6, was involved in implementing the scoring system of the evidence
Statistical analysis
This meta-analysis was performed using Stata 12.0 (Stata Corporation, College Station, TX, USA). A fixed-effects model with Mantel-Haenszel method was used to pool data and synthesize the quantitative analysis when the heterogeneity is not evident. Cochran Q test and I2 statistic were used to assess the heterogeneity. Significant heterogeneity is identified when the P value is less than 0.1 or I2 is more than 50%. Relative risks (RRs) and the corresponding 95% confidence intervals (CIs) were used to compare the efficacy and safety of SAPT with that of DAPT with two-tailed P value (defined statistical significance when P<0.05). Furthermore, a subgroup analysis was conducted to evaluate the results from different types of studies. The publication bias of the primary outcome was investigated by visual estimation of funnel plots.
Results
Characteristics of included studies
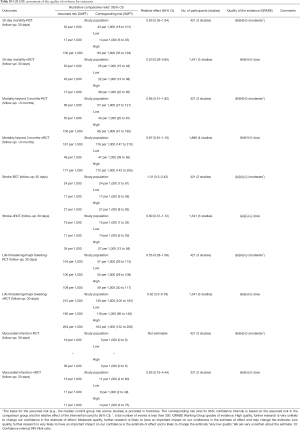
As shown in the Figure 1, we screened 918 records based on title or abstract and there were eight studies (10-17) which met the inclusion criteria defined in the current study and were thus included in our final analysis: three RCTs and five observational studies. Furthermore, out of the five observational studies, three studies (12,14,15) are propensity score matching (PSM) analyses. Overall, using data after PSM, our meta-analysis included a total of 2,489 patients (1,149 on SAPT, 1,340 on DAPT). The characteristics of the eight studies are described in Table 1 and the clinical features of the patients are listed in Table 2. The inclusion and exclusion criteria of the included studies are shown in Table S1.
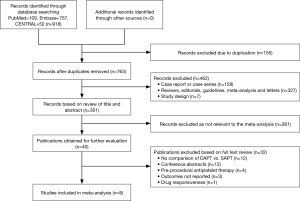
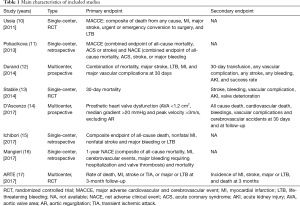
Full table
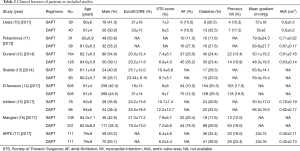
Full table
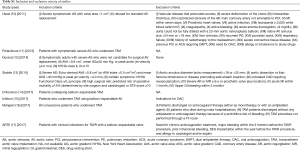
Full table
The main access of TAVR used in studies was transfemoral approach, in addition to other approaches including apex, aorta, subclavian, carotid and iliac arteries. The duration of DAPT in these studies mostly ranged from 3 to 6 months, consistent with the recommendation of current guidelines, while 1 month of clopidogrel and aspirin was scheduled in a PSM study (12). Moreover, thienopyridine such as ticlopidine was used in two studies (13,15). SAPT with lifelong low-dose acetylsalicylic acid (ASA) was adopted in three studies, whereas some other studies used ASA for 6 months (11,16,17).
The duration of follow-up in most studies was 30 days. In a retrospective analysis of registry, the median follow-up was 45.0±14.0 months (14). The 30-day outcomes were documented in six studies (10-14,17), whereas 3 studies (10,11,13) provided data at 6 months, and 1 study (17) at 90 days. Two studies (15,16) only reported the 1-year outcomes.
All-cause mortality
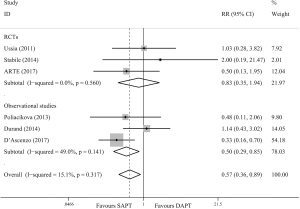
Six trials (10-14,17) reported the data on all-cause mortality at 30 days. As shown in Figure 2, SAPT lead to a significant decline compared with DAPT in terms of overall effect (RR =0.57; 95% CI, 0.36–0.89; P=0.014). There was no significant difference between the two strategies in RCTs (RR =0.83; 95% CI, 0.35–1.94; P=0.662) and observational studies showed significant subtotal effect (RR =0.50; 95% CI, 0.29–0.85; P=0.010).
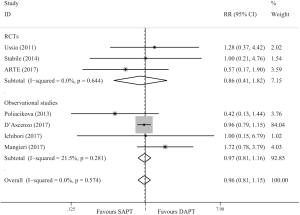
Seven trials (10,11,13-17) reported the data on all-cause mortality beyond 3 months. As shown in Figure 3, the death beyond 3 months is similar for both SAPT and DAPT groups (RR =0.96; 95% CI, 0.81–1.15; P=0.664). In the subgroup analysis, similar results were obtained in RCTs (RR =0.86; 95% CI, 0.41–1.82; P=0.702) and observational studies (RR =0.97; 95% CI, 0.81–1.16; P=0.735).
Stroke
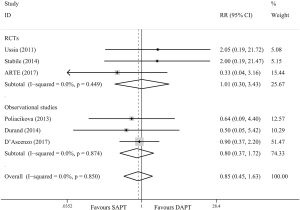
In six studies (10-14,17) involving 1,962 patients, 30-day stroke (major and minor) occurred in 36 patients (1.8%) and no significance was observed (RR =0.85; 95% CI, 0.45–1.63; P=0.631) in either RCTs (RR =1.01; 95% CI, 0.30–3.43; P=0.990) or observational studies (RR =0.80; 95% CI, 0.37–1.72; P=0.567) (Figure 4).
Life-threatening or major bleeding
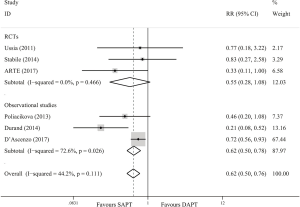
Life-threatening or major bleeding at 30 days was reported in 294 patients (14.9%) by pooling data from the six studies (10-14,17). SAPT showed a benefit over DAPT in reducing the risk of life-threatening or major bleeding (RR =0.62; 95% CI, 0.50–0.76; P=0.000). The consistent results were obtained in observational studies (RR =0.62; 95% CI, 0.50–0.78; P=0.000) whereas no significance was observed in RCTs (RR =0.55; 95% CI, 0.28–1.08; P=0.082) (Figure 5).
Myocardial infarction
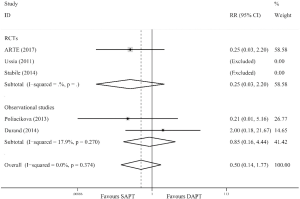
The 30-day spontaneous MI was documented by five studies (10-13,17). As shown in Figure S1, the incidence of MI was not different in SAPT vs. DAPT (RR =0.50; 95% CI, 0.14–1.77; P=0.281). Similarly, there was no difference in subgroup analysis for RCTs (RR =0.25; 95% CI, 0.03–2.20; P=0.212) and observational studies (RR =0.85; 95% CI, 0.16–4.44; P=0.843).
Quality assessment
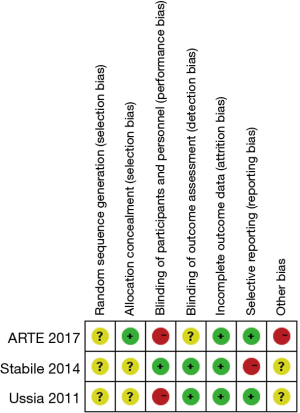
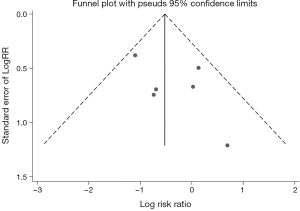
As shown in the Figure S2, all RCTs have unclear bias for random sequence generation without giving sufficient messages. And only one trial (17) reported using random block size to conceal treatment allocation. Two of the three trials are open-labeled studies (10,13). For reporting bias, one study (13) did not report all outcomes. Furthermore, the ARTE trial (17) was prematurely stopped after the inclusion of 74% of the planned study population thus it was deemed to have a high risk of other biases. According to the Newcastle-Ottawa scale (Table S2), most observational studies were found to be of good quality. The summary of quality assessment for outcomes by GRADE was shown in Table S3 for both RCTs and observational studies. The level of the evidence for RCTs was downgraded due to the limited number of events. The funnel plots of 30-day mortality were symmetrical by visual estimation thus there was no evident publication bias (Figure S3). No significant heterogeneity was observed between studies for each outcome.

Full table
Full table
Discussion
This meta-analysis of SAPT versus DAPT in the patients undergoing TAVR enrolled 2489 participants from three randomized trials and five observational studies. Our results show that SAPT (vs. DAPT) may decrease the incidence of death at 30 days, with a reduced risk of major/life-threatening bleeding. Furthermore, SAPT is noninferior to DAPT in terms of all-cause mortality beyond 3 months. For other secondary outcomes, there is no significant difference between the two regimens with respect to 30-day stroke and spontaneous MI. These data thus suggest that compared with DAPT, SAPT may be used as a safer post-TAVR strategy to improve the prognosis of patients.
Stroke and hemorrhage are two common complications after TAVR. The antiplatelet therapy is a two-edged sword for clinicians to balance the risk of ischemia and bleeding events in the postoperative management of patients. Patients with aortic valve stenosis often have elder age and multiple comorbidities such as atrial fibrillation (AF) or coronary artery disease, which undoubtedly increases the complexity of applying antiplatelet regimens. Although 6-month DAPT is recommended for those people without indication for anticoagulation by current guidelines (1), this recommendation is an empirical strategy.
There is still a controversy about antiplatelet regimens after TAVR. Previous meta-analyses conducted by Aryal et al. (5) and Gandhi et al. (6) indicated that the effect of SAPT is not inferior to DAPT and there is even a tendency to reduce bleeding events. On the contrary, Verdoia et al. (18) supported the use of DAPT for the decreased mortality without increasing the risk of bleeding. To investigate the optimal antiplatelet strategy after TAVR, we therefore performed this updated meta-analysis including ARTE trial (17) and other recent observational studies. We only included the studies which directly compare the two groups, avoiding the interference from anticoagulation. Considering the limited number of RCTs, we also pooled the data from observational studies and performed subgroup analysis to observe the potential impact. Compared with previous meta-analyses, this research retrieved eight studies including more than 2,000 participants. Further, we also assessed the data beyond 3 months and used GRADE approach to evaluate the quality of outcomes, which has not been applied in previous analyses.
Our results show a significant benefit of SAPT over DAPT on all-cause death and bleeding events at 30 days. The decline of all-cause mortality can benefit from the decreased major or life-threatening bleeding events. Meanwhile, there was no significant difference observed in terms of 30-day stroke, MI as well as mortality beyond 3 months during the comparison of SAPT and DAPT. These findings indicated that, compared with the 6-month DAPT recommended in the previous publications, SAPT may be an appropriate strategy after TAVR. However, it is essential to take into account that the sample size is still relatively small and data from large randomized trials are required to evaluate the two strategies. The POPular-TAVI (Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic Valve Implantation; NCT02247128) cohort A is an ongoing large randomized study designed to assess the 1-year outcome between DAPT and aspirin alone, which will provide further investigation in patients without an indication for oral anticoagulant (OAC).
There are several limitations in our analysis. In the first place, despite the latest trials included, our sample size was relatively small due to a lack of relevant published studies, which would limit the statistical power of this analysis and the ability to observe statistically significant effects. In addition, few published studies reported the clinical outcomes beyond 30 days. As a result, the safety and efficacy of the two regimens in terms of long-term follow-up cannot be well observed. However, it was noted that there was no evident heterogeneity and publication bias in this analysis.
Conclusions
In conclusion, our findings suggest that SAPT after TAVR may have less 30-day mortality than DAPT by means of reducing the incidence of bleeding, with no increased risk of stroke and MI compared with DAPT. Further large-scale randomized controlled studies will elucidate the uncertainty of antiplatelet regimens after TAVR and establish the optimal approach to minimizing ischemic and bleeding risks.
Search strategy for PubMed, Embase and CENTRAL
PubMed: 109
Embase: 757
CENTRAL: 52
Acknowledgements
Funding: This work was supported by Jiangsu Provincial Key Research and Development Program (BE2016785) and Jiangsu Provincial Key Medical Discipline (ZDXKA2016023).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study did not involve any experiment on humans or animals, thus ethical approval was not necessary.
References
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2017;376:1321-31. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [Crossref] [PubMed]
- Aryal MR, Karmacharya P, Pandit A, et al. Dual versus single antiplatelet therapy in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis. Heart Lung Circ 2015;24:185-92. [Crossref] [PubMed]
- Gandhi S, Schwalm JD, Velianou JL, et al. Comparison of Dual-antiplatelet Therapy to Mono-antiplatelet Therapy After Transcatheter Aortic Valve Implantation: Systematic Review and Meta-analysis. Can J Cardiol 2015;31:775-84. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403-18. [Crossref] [PubMed]
- GA Wells, B Shea, D O'Connell, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2008. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Ussia GP, Scarabelli M, Mule M, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardiol 2011;108:1772-6. [Crossref] [PubMed]
- Poliacikova P, Cockburn J, de Belder A, et al. Antiplatelet and antithrombotic treatment after transcatheter aortic valve implantation - comparison of regimes. J Invasive Cardiol 2013;25:544-8. [PubMed]
- Durand E, Blanchard D, Chassaing S, et al. Comparison of two antiplatelet therapy strategies in patients undergoing transcatheter aortic valve implantation. Am J Cardiol 2014;113:355-60. [Crossref] [PubMed]
- Stabile E, Pucciarelli A, Cota L, et al. SAT-TAVI (single antiplatelet therapy for TAVI) study: a pilot randomized study comparing double to single antiplatelet therapy for transcatheter aortic valve implantation. Int J Cardiol 2014;174:624-7. [Crossref] [PubMed]
- D'Ascenzo F, Benedetto U, Bianco M, et al. Which is the best antiaggregant or anticoagulant therapy after TAVI? A propensity-matched analysis from the ITER registry. The management of DAPT after TAVI. EuroIntervention 2017;13:e1392-400. [Crossref] [PubMed]
- Ichibori Y, Mizote I, Maeda K, et al. Clinical Outcomes and Bioprosthetic Valve Function After Transcatheter Aortic Valve Implantation Under Dual Antiplatelet Therapy vs. Aspirin Alone. Circ J 2017;81:397-404. [Crossref] [PubMed]
- Mangieri A, Jabbour RJ, Montalto C, et al. Single-Antiplatelet Therapy in Patients with Contraindication to Dual-Antiplatelet Therapy After Transcatheter Aortic Valve Implantation. Am J Cardiol 2017;119:1088-93. [Crossref] [PubMed]
- Rodes-Cabau J, Masson JB, Welsh RC, et al. Aspirin Versus Aspirin Plus Clopidogrel as Antithrombotic Treatment Following Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve: The ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) Randomized Clinical Trial. JACC Cardiovasc Interv 2017;10:1357-65. [Crossref] [PubMed]
- Verdoia M, Barbieri L, Nardin M, et al. Dual Versus Single Antiplatelet Regimen With or Without Anticoagulation in Transcatheter Aortic Valve Replacement: Indirect Comparison and Meta-analysis. Rev Esp Cardiol (Engl Ed) 2018;71:257-66. [Crossref] [PubMed]