Application of alveolar recruitment strategy and positive end-expiratory pressure combined with autoflow in the one-lung ventilation during thoracic surgery in obese patients
Introduction
One-lung ventilation (OLV) is a very important ventilation method for anesthetic management during thoracic surgery, which can prevent the blood and secretions of the sick-side lung from entering into the healthy-side lung to keep the airway open, avoid cross infection, and be good for the operation. In further studies, pulmonary complications, such as pneumonia pyothorax, and atelectasis, have been constantly reported. A large number of literatures (1-8) have suggested that mechanical ventilation can result in ventilator-induced lung injury (VILI). At present, it has been considered that VILI is caused by the following two aspects. One aspect is that the excessively high end-inspiratory pressure or volume can lead to increased pressure across the alveolar or alveolar overexpansion, causing barotrauma or volutrauma (9), while the other aspect is that at the end of expiration, the pressure across the alveolar decreases under critical pressure, which induces the alveoli to open, and the repeated opening and closing of the alveoli can cause shearing injury. Some studies (10,11) have indicated that shearing injury plays an important role in the VILI. The key to reduce the occurrence of VILI is that during the mechanic ventilation, the pressure at the airway and alveoli should be reasonably controlled to avoid unstable conditions of the alveoli.
Obesity has become a global concern. Obese patients usually suffer from many kinds of diseases. For obese patients, thorax compliance decreases, the diaphragm increases, and functional residual volume, vital capacity and total lung capacity decreases. The decrease in thorax compliance can easily lead to excessive high airway pressure, which can result in lung damage. For obese patients, oxygen consumption and CO2 production both increases, and changes in position has can lead to obvious impacts on lung capacity. When obese patients are in the lateral position, functional residual volume further decreases, causing thorax compliance to further decrease and the ventilation/perfusion ratio (V/Q) to be imbalanced. During the OLV, lung damage and hypoxemia can easily occur (12).
Therefore, during ventilation for obese patients, the release of pro-inflammatory cytokines can easily increase and result in lung damage (13), and during the general anesthesia, complications, such as atelectasis and lung infections (14,15), can easily occur. Obesity has been an independent risk factor for lung damage caused by mechanical ventilation (16). Due to the lung damage caused by mechanic ventilation during the operation, on the 1st day after operation, hypoxemia can easily occur (17,18).
During the OLV in thoracotomy, volume control ventilation (VCV) and pressure-controlled ventilation (PCV) are the two ventilation methods usually used. However, VCV can produce excessively high airway pressure, which can damage the lung, and cause an uneven gas distribution in the lungs (19). Although PVC is better matched with a patient’s respiratory physiology, and can reduce airway pressure and atelectasis, it can induce lung hypoventilation and hypoxemia due to pulling and stretching during the operation (5). Therefore, it is very important to find a more reasonable pulmonary ventilation model.
Autoflow ventilation is based on real-time airway resistance and respiratory system compliance, and the medical ventilator adjusts the air flow to ensure that the air carried to the lungs is at the lowest pressure level. It is not an independent ventilation model, but an assisted ventilation technique that is an expansion of VCV function, which should be combined with VCV. Autoflow ventilation can prevent the hypoventilation caused by surgery and clear reduce complications, and has been approved as an effective ventilation model by critical patients (20). However, there are no relevant reports for OLV during thoracic surgery.
During OLV, solely small tidal volume ventilation cannot decrease the occurrence of sheering injury of the lungs, which is close to alveolar trapping and re-expansion (6,21). Fernandez-Bustamante et al. (16) noted that for obese patients with OLV, the ventilation-side lung more easily suffered from atelectasis, and that positive end-expiratory pressure (PEEP) was the most effective approach to keep the alveoli open, which could reduce the collapse of the alveoli (7,8).
The atelectasis of the ventilation-side lung usually occurs in the patients with general anesthesia and mechanical ventilation, and the atelectasis can increase the occurrence of hypoxemia and lung infection (22). The study conducted by Talab et al. (23) reported that alveolar recruitment strategy (ARS) was a best way to avoid atelectasis in obese patients. The appropriate PEEP was given before OLV and after ARS, which can reduce lung damage and improve oxygen index (OI). However, there are no relevant reports for obese patients.
The present study aims to explore the value of the application of ARS and PEEP combined with an autoflow ventilation model in obese patients with OLV during thoracic surgery.
Methods
The present study was approved by the Ethics Committee of Beijing Chest Hospital Affiliated to Capital Medical University. A written informed consent was obtained from each patient or their families.
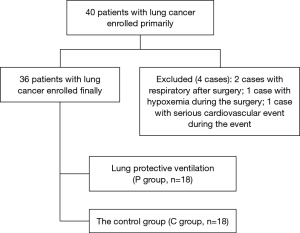
The present study was a randomized controlled study. A total of 36 obese patients, who would experience elective lobectomy from June 1, 2017 to March 31, 2018, were enrolled. The age of these patients ranged within 39–74 years old, their body mass index (BMI) was 30–40 kg/m2, and they belonged to ASA II–III grade. In the latest 1 week, these patients did not have a history of infection, did not use cortical hormone, and did not receive radiation therapy and chemotherapy. Furthermore, they had normal pulmonary function or only slight ventilatory disorders, and did not have liver and kidney disorders. Exclusion criteria: Before the operation, these patients were diagnosed with obstructive sleep apnea hypopnea syndrome, had a history of pneumothorax or right heart failure, the measurement of arterial oxygen before the operation was lower than 90%, and bleeding during the operation was more than 400 mL.
According to the random number table, these patients were divided into two groups: control group (C group) and lung protective ventilation group (P group). The tidal volume of patients was 9 mL/kg under two-lung ventilation, and 7 mL/kg under OLV (tidal volume calculation according to standard weight). Patients with OLV in the C group were given the VCV model. In the P group, autoflow ventilation was given, pulmonary re-expansion was given once before OLV, and 40 mmHg of airway pressure was given at less than 40 seconds before OLV, but 7 mmHg of PEEP was not given upon expiration. Electrocardiogram (ECG), blood pressure (BP) of the radial artery, central venous pressure and SPO2 were routinely monitored after the patients entered the operation room.
Anesthesia induction: During the operation, 0.3 µg/kg of sufentanil and 0.1 mg/kg of midazolam were slowly injected into the vein in turns, the target-controlled infusion (TCI) of propofol was performed using a micro syringe pump, and the concentration of the blood plasma target was 3.0–4.0 µg/mL. After patients lost their consciousness, 0.2 mg/kg of cisatracurium was intravenously injected, oxygen flow was 5 L/min, oxygen was inhaled through the facemask to remove nitrogen, muscle relaxation monitoring was started [train-of-four (TOF) stimulation], and connection and scaling were performed before anesthesia induction. After T1 disappeared, a tracheal tube was inserted into the left-sided double lumen endobronchial tube, and the right location of tracheal tube and cuff was identified by fiberoptic bronchoscopy and fixed. The temperature of the nasopharynx was monitored, and the body temperature of patients was maintained using an electric blanket. The mechanical ventilation was performed by the anesthesia respirator.
Maintenance of anesthesia: After fiberoptic bronchoscopy at the lateral position was used again to ensure the right location, TLV was given. According to the experiment design, the ventilation parameters were respectively set in the two groups: FiO2 =1.0, fresh gas flow: 2.5 L/min, expiration and inspiration ratio: 1:1.5, volume controlled ventilation, and the adjustment of respiratory rate in the range of PetCO2: 35–45 mmHg (1 kPa =7.5 mmHg); TCI of propofol, 2.5 µg/mL of blood plasma target, intravenous injection of cisatracurium besylate and sufentanil upon conditions during the operation, maintenance of muscle twitch that disappeared during T1–T4, keeping BIS between 40–60, and electric blanket temperature holding between 38–43 °C. During the anesthesia, HR60-100 times/minute was maintained, and the MAP fluctuation range was less than 20% of the basal value. Under the conditions of excluding the influences of anesthesia depth, when MAP was more than 20% of the basal value and lasted more than 1 minute, 12.5 mg of urapidil was injected into the vein. Under the conditions of excluding the influences of anesthesia depth, when MAP was less than 20% of the basal value and lasted for more than 1 minute, and the infusion of 50 mL of fluids completed within 5–10 minutes was ineffective, 6 mg of ephedrine was injected into the vein. In the case of HR ≤50 times/minute or HR ≥100 times/minute, 0.2 mg of atropine or 5 mg of esmolol was injected into the vein, respectively. The above vasoactive drugs could be repeatedly used. If the above method could not keep the patient circulation stable, the patient was dropped out of the present study.
The bypass side-stream monitoring system was used to observe the ventilation of patients, and Ppeak, Pplat and Cdyn were recorded every 5 minutes. The above data at T1, T2 and T3 was collected as the experimental data. Arterial and venous blood at T1, T2, T3, T4 and T5 was collected, and a blood gas analysis was performed to record the SPO2, PaCO2 and PaO2, and calculate the Qs/Qt(%). Venous blood was drawn at T1 and T5, and the concentrations of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were detected by enzyme linked immunosorbent assay.
Observation indicators: Qs/Qt(%), OI, PaCO2 at T1, T2, T3, T4 and T5; Ppeak, Pplat and Cdyn at T1, T2 and T3; the concentrations of IL-6 and TNF-α in venous blood at T1 and T5; clinical pulmonary infection score (CPIS) at the 1st day and 7th day after the operation (21).
Statistical analysis
SPSS 19.0 was used, measurement data was expressed as mean ± standard deviation (SD), intra-group comparison was performed by repeated measure of ANOVA, t-test was adopted for inter-group data, and P<0.05 was considered statistically significant. If there was heterogeneity of variance, the rank-sum test was used, and P<0.05 was considered statistically significant. Fisher’s precise test was used for enumeration data. P<0.05 was considered statistically significant.
Results
Two cases required ventilator support after the operation in the P group and C group. A case had intraoperative hypoxemia in the P group, while a case had severe cardiovascular events during operation in the P group.
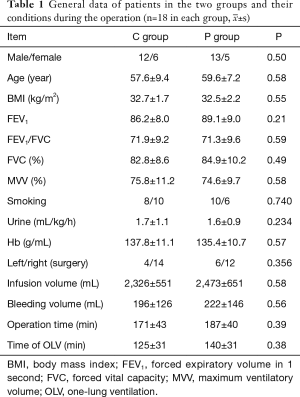
Compared the general data, such as gender, age, BMI, pulmonary function, hemoglobin (Hb), transfusion volume, bleeding volume during the operation, and time of OLV between the two groups, the difference was not statistically significant (P>0.05, Table 1).
 )
) Full table
Compared with T1, at T2 and T3, Cdyn decreased and Pplat and Ppeak increased in both groups (P<0.05). Compared with the C group, at T2 and T3, Pplat and Ppeak decreased in the P group (P<0.05, Table 2).
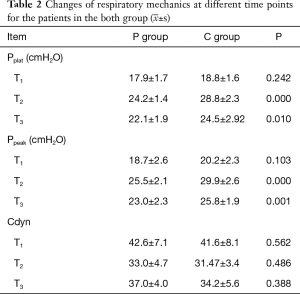
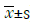 )
) Full table
Compared with T1, at T2 and T3, Qs/Qt(%) increased, while OI decreased (P<0.05) in both groups. Compared with the C group, at T2 and T3, OI increased in the P group. Compared with the C group, at T2, T3 and T4, PaCO2 and Qs/Qt(%) decreased in the P group (P<0.05, Table 3).
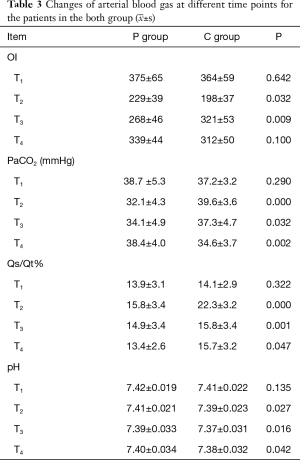
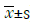 )
) Full table
Compared with T1, at T3, the concentrations of IL-6 and TNF-α increased in both groups (P<0.05). Compared with the C group, the concentrations of IL-6 and TNF-α in the P group decreased (Table 4, Figure 1).
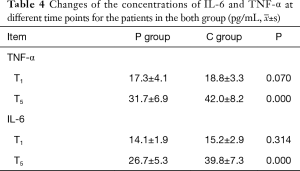
 )
) Full table
There was no difference in CPIS at the 1st day and 7th day after the operation (P>0.05, Table 5).
Discussion
The present study revealed that patients in the P group maintained a lower Ppeak and platform during the operation, lower Qs/Qt and better OI during the operation and at 6 hours after the operation, and avoided the accumulation of CO2 during and after the operation, and blood pH was closer to the normal value after operation. However, the infection scores in the P group did not improve on the 1st day and 7th day after the operation.
Ppeak is mainly determined by the tidal volume and inspiratory duration. In the case of Ppeak>40 cmH2O, the occurrence of VILI sharply increased (24). Pplat was correlated to static lung compliance (25), and was positively correlated with barotrauma. When Pplat was less than 25 cmH2O, barotrauma hardly occurred. However, when Pplat was more than 29 cmH2O, the incidence of barotrauma clearly increased (21). High Pplat induces alveolar wall overstretching, which causes damage and increases the permeability of the alveolar epithelium and vascular endothelium, leading to high permeability pulmonary edema and damage.
The present study indicated that at T2 and T3, compared with the C group, Pplat obviously decreased in the P group. During the OLV, three patients had a Pplat of >30 cmH2O, and after properly bringing down the tidal volume, two patients continued to have a Pplat of >25 cmH2O. The reason may be that in the P group, PEEP combined with autoflow ventilation could not only better maintain the alveoli recruitment maneuver, but also prevent alveolar collapse at the end of expiration. Thus, the occurrence of pulmonary collapse and damage can be avoided, Cdyn is improved, Pplat decreases, and the lung damage is alleviated.
In the P group, during the OLV, Qs/Qt has remained low. During the operation, two patients in the C group experienced hypoxemia, which both occurred within 30 minutes of OLV and lasted for <10 minutes. In the P group, alveolar collapse and atelectasis were effectively prevented, end-expiratory alveoli were kept relatively open, ARS induced the collapsed alveoli to open again, and OI was improved, which prevented hypoxemia. This is consistent with the report of Aldenkortt et al. (26), which noted that ARS could reduce the occurrence of hypoxemia during the operation.
Massive inflammatory mediators were produced by neutrophils, alveolar macrophage and alveolar epithelial cells when they suffered the mechanic damage, causing tissue damage. Massive inflammatory mediators, inflammatory cells and adhesion molecules were released in blood through pulmonary circulation. IL-6 is an important indicator that reflects the tissue injury degree in the early stage (27,28), and TNF-α is a sensitive indicator that reflects tissue injury degree (5). Testing the concentrations of TNF-α and IL-6 can be used to determine the condition of inflammation in the lung.
At T5, the concentrations of IL-6 and TNF-α obviously increased in both groups, since thoracic surgery and mechanic ventilation can damage the tissue, and induce TNF-α and IL-6 to increase. Compared with the C group, the concentrations of IL-6 and TNF-α decreased in the P group, suggesting that lung protective ventilation decreased the generation of inflammatory mediators, thereby alleviating lung damage. However, on the 1st day and 7th day after the operation, there was no significant difference in CPIS between the two groups, which indicate that lung protective ventilation cannot reduce the incidence of pulmonary infection after surgery.
There were also some limitations in the present study. First, this is not a double-blinded study, and the anesthetist knew about the condition of all patients. Thus, bias could not be avoided. Second, for monitoring cytokines in venous blood, it would take certain period of time to produce inflammatory cytokines and release these in the blood. Thus, it would be more accurate to use alveolar lavage fluid to reflect the lung injury degree. Third, the sample was small, and bias could not be avoided. Lastly, but not the least, the age range was wide, and the decrease in lung function in for elderly patients can inevitably have an impact on the results.
Conclusions
In conclusion, the ventilation model of ARS and PEEP combined with autoflow can better reduce airway pressure and the production of injurious inflammatory cytokines in blood in obese patients. Furthermore, it can reduce Qs/Qt during and at 6 hours after the thoracotomy, improve OI and maintain the acid-base balance of the internal environment, which may be provided during clinical work. These bring new enlightenment and needs to be clarified through further studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present study was approved by the Ethics Committee of Beijing Chest Hospital Affiliated to Capital Medical University (IRB number: 2016-30) and written informed consent was obtained from all patients.
References
- Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013;118:1307-21. [Crossref] [PubMed]
- PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, Gama de Abreu M, et al. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014;384:495-503. [Crossref] [PubMed]
- Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;308:1651-9. [Crossref] [PubMed]
- Fiorelli S, Defraia V, Cipolla F, et al. Short-term one-lung ventilation does not influence local inflammatory cytokine response after lung resection. J Thorac Dis 2018;10:1864-74. [Crossref] [PubMed]
- Tan J, Song Z, Bian Q, et al. Effects of volume-controlled ventilation vs. pressure-controlled ventilation on respiratory function and inflammatory factors in patients undergoing video-assisted thoracoscopic radical resection of pulmonary carcinoma. J Thorac Dis 2018;10:1483-9. [Crossref] [PubMed]
- Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2007;175:160-6. [Crossref] [PubMed]
- Rozé H, Lafargue M, Ouattara A. Case scenario: Management of intraoperative hypoxemia during one-lung ventilation. Anesthesiology 2011;114:167-74. [Crossref] [PubMed]
- Della Rocca G, Coccia C. Acute lung injury in thoracic surgery. Curr Opin Anaesthesiol 2013;26:40-6. [Crossref] [PubMed]
- Barbosa FT, Castro AA, de Sousa-Rodrigues CF. Positive end-expiratory pressure (PEEP) during anaesthesia for prevention of mortality and postoperative pulmonary complications. Cochrane Database Syst Rev 2014.CD007922. [PubMed]
- Shen Y, Zhong M, Wu W, et al. The impact of tidal volume on pulmonary complications following minimally invasive esophagectomy: a randomized and controlled study. J Thorac Cardiovasc Surg 2013;146:1267-73; discussion 1273-4. [Crossref] [PubMed]
- Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139:530-7. [Crossref] [PubMed]
- Vaughan RW, Wise L. Postoperative arterial blood gas measurement in obese patients: effect of position on gas exchange. Ann Surg 1975;182:705-9. [Crossref] [PubMed]
- de Souza ABF, Chírico MTT, Cartelle CT, et al. High-Fat Diet Increases HMGB1 Expression and Promotes Lung Inflammation in Mice Subjected to Mechanical Ventilation. Oxid Med Cell Longev 2018;2018:7457054. [Crossref] [PubMed]
- Sequeira TCA. BaHammam AS, Esquinas AM. Noninvasive Ventilation in the Critically Ill Patient With Obesity Hypoventilation Syndrome: A Review. J Intensive Care Med 2017;32:421-8. [Crossref] [PubMed]
- Karnatovskaia LV, Lee AS, Bender SP, et al. US Critical Illness and njury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). Obstructive sleep apnea, obesity, and the development of acute respiratory distress syndrome. J Clin Sleep Med 2014;10:657-62. [PubMed]
- Fernandez-Bustamante A, Hashimoto S, Serpa Neto A, et al. Perioperative lung protective ventilation in obese patients. BMC Anesthesiol 2015;15:56. [Crossref] [PubMed]
- Rosenberg J. Sleep disturbances after non-cardiac surgery. Sleep Med Rev 2001;5:129-37. [Crossref] [PubMed]
- Rosenberg J, Rasmussen GI, Wøjdemann KR, et al. Ventilatory pattern and associated episodic hypoxaemia in the late postoperative period in the general surgical ward. Anaesthesia 1999;54:323-8. [Crossref] [PubMed]
- Pu J, Liu Z, Yang L, et al. Applications of pressure control ventilation volume guaranteed during one-lung ventilation in thoracic surgery. Int J Clin Exp Med 2014;7:1094-8. [PubMed]
- Lasocki S, Labat F, Plantefeve G, et al. A long-term clinical evaluation of autoflow during assist-controlled ventilation: a randomized controlled trial. Anesth Analg 2010;111:915-21. [PubMed]
- Tusman G, Böhm SH, Suarez-Sipmann F. Dead space during one-lung ventilation. Curr Opin Anaesthesiol 2015;28:10-7. [Crossref] [PubMed]
- Alfille PH. Using recruitment maneuvers to decrease tidal volumes during one-lung ventilation. Anesthesiology 2011;114:1009-10. [Crossref] [PubMed]
- Talab HF, Zabani IA, Abdelrahman HS, et al. Intraoperative ventilatory strategies for prevention of pulmonary atelectasis in obese patients undergoing laparoscopic bariatric surgery. Anesth Analg 2009;109:1511-6. [Crossref] [PubMed]
- van der Werff YD, van der Houwen HK, Heijmans PJ, et al. Postpneumonectomy pulmonary edema. A retrospective analysis of incidence and possible risk factors. Chest 1997;111:1278-84. [Crossref] [PubMed]
- Marini JJ, Ravenscraft SA. Mean airway pressure: physiologic determinants and clinical importance--Part 2: Clinical implications. Crit Care Med 1992;20:1604-16. [Crossref] [PubMed]
- Aldenkortt M, Lysakowski C, Elia N, et al. Ventilation strategies in obese patients undergoing surgery: a quantitative systematic review and meta-analysis. Br J Anaesth 2012;109:493-502. [Crossref] [PubMed]
- Schädler D, Pausch C, Heise D, et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS One 2017;12:e0187015. [Crossref] [PubMed]
- Rentzsch I, Santos CL, Huhle R, et al. Variable stretch reduces the pro-inflammatory response of alveolar epithelial cells. PLoS One 2017;12:e0182369. [Crossref] [PubMed]