
Extracellular matrix proteins as circulating biomarkers for the diagnosis of non-small cell lung cancer patients
The extracellular matrix (ECM) is composed of a dynamic network of macromolecules which assemble into three-dimensional structures functioning as ligands for cellular integrins or acting as a scaffold for soluble cytokines and growth factors (1). Whereas the role of ECM in the regulation of physiological tissue development and homeostasis has been already characterized, the study of the involvement of the ECM in cancer progression is a subject of growing interest. Abnormal changes in the amount and composition of the ECM, consisting in increased deposition and altered organization of ECM proteins or in enhanced post-translational modifications, are commonly observed in cancer. Altered ECM proteins can promote cell survival and proliferation, and can support cell migration and invasion. Moreover, ECM proteins can also induce tumor-associated inflammation and angiogenesis, thus contributing to cancer progression (1). As a consequence of deregulated ECM remodeling in cancer, an increased number of protein-specific fragments deriving from ECM turnover are released into blood. Such molecules can be potentially used as circulating biomarkers useful for clinical diagnosis and monitoring of cancer patients (2).
As one of the earlier events during tumorigenesis is the generation of cancer-associated fibroblasts (CAFs), which mainly contribute to abnormal ECM remodeling (1), Andriani and colleagues reasoned that ECM proteins released by CAFs into circulation can be potentially used as biomarkers for the early detection of lung cancer. To verify this hypothesis, they compared microarray gene expression profiles of normal fibroblasts (NFs) and CAFs derived from non-small cell lung cancer (NSCLC) patients (3). Gene enrichment analysis showed that genes related to ECM deposition and remodeling were significantly enriched in CAFs. They focused their attention on the ECM3 signature, a group of 58 genes coding for macromolecules involved in ECM remodeling with prognostic value in breast cancer, and demonstrated that 11 out of the 58 genes were significantly differentially expressed between NFs and CAFs. Among the genes belonging to the ECM panel, the authors characterized the role of three proteins whose expression was upregulated in CAFs compared to NFs: collagen type XI α1 (COL11A1) and collagen type X α1 (COL10A1), that were the two mostly upregulated genes, and secreted protein acidic and rich in cysteine (SPARC).
Circulating COL11A1 has been already proposed as a diagnostic marker in cancer. In fact, increased plasma levels of COL11A1 have been reported in breast cancer patients and its levels were able to discriminate between patients with breast carcinoma and benign disease (4). In the paper by Andriani it was shown that the upregulation of COL11A1 mRNA in CAFs did not correspond to a significant increase of intracellular and secreted protein expression levels. Moreover, the authors did not find any difference in plasma circulating amounts of the protein between lung cancer patients and healthy heavy-smokers controls. Again, no changes of COL11A1 expression levels were observed between patients subjected or not to chemotherapy treatment before surgery. Finally, no significant association between COL11A1 plasma levels and overall survival of NSCLC patients was found. Based on these preliminary data, the measurement of COL11A1 in plasma seems not to be useful for early diagnosis of NSCLC. Nevertheless, COL11A1 has been reported to be overexpressed in tumor tissue samples from NSCLC patients, and its expression levels have been correlated to tumor size and stage and to presence of lymph node metastases (5). Therefore, it can be supposed that the evaluation of intratumoral expression of COL11A1 has a potential clinical utility as prognostic biomarker for lung cancer patients.
Also for COL10A1 an increased release into circulation has already been reported in cancer, in particular in breast and colon tumors, and such factor has been proposed as a promising biomarker for early detection of colon cancer patients (4,6). One of the advantages of the use of COL10A1 as a cancer biomarker is that it is expressed only during endochondral ossification and it is normally absent in healthy adult tissues (7). Andriani and colleagues demonstrated that COL10A1 protein expression and secretion were increased in CAFs compared to NFs and that its plasma levels were significantly higher in NSCLC patients than in controls, particularly in females. Nevertheless, circulating COL10A1 levels showed no significant association with disease status, overall survival and chemotherapy response of NSCLC patients. Based on the data reported in the paper by Andriani and colleagues, no definitive conclusions can be drawn on the potential use of COL10A1 as a diagnostic marker of lung cancer. Further studies on a larger cohort of patients are required to strengthen the significance of increased plasma levels of COL10A1 in NSCLC, with a particular focus on female patients.
The most encouraging results were obtained from the analysis of SPARC. SPARC is a secreted matricellular glycoprotein that directly participates in ECM assembly and turnover, through the regulation of different processes, such as metalloproteinases secretion, growth factors signaling and cell-ECM interaction (8). Intriguingly, SPARC has been shown to have both oncogenic and tumor suppressor properties in different types of human cancers (8). There is evidence that increased serum levels of SPARC can be used as a diagnostic marker for pancreatic cancer, melanoma and hepatocellular carcinoma (9-11).
In the paper by Andriani and colleagues, SPARC overexpression in CAFs was confirmed at both intracellular and secreted protein levels. Moreover, circulating amounts of SPARC resulted significantly higher in NSCLC patients compared to controls and were associated with disease status. The OD value of 0.587, resulting from the measurement of plasma levels of SPARC by ELISA assay, proved to be the optimal cutoff to discriminate patients from healthy controls, corresponding to a specificity of 78.9% and a sensitivity of 64.4%. SPARC plasma levels did not differ significantly among tumor stages and were not associated to the overall survival of NSCLC patients, indicating that SPARC release into blood is an early event during lung tumorigenesis and that it is independent from the prognosis. The lack of correlation between SPARC plasma levels and the overall survival of NSCLC patients observed by Andriani and coauthors appears to be in contrast with previous data reporting that high expression of SPARC in NSCLC stroma is associated to poor prognosis (12). Conversely, high expression of the protein within tumor tissue has been associated to better survival of NSCLC patients (13). Therefore, it seems that the role of SPARC in lung cancer microenvironment and its correlation with patients outcome is dependent on the cellular context, whereas SPARC circulating levels, resulting from the secretory activity of both tumor and stroma cell types, can be used uniquely as an indicator of the disease status.
Finally, preliminary data in the paper by Andriani showed a slight but statistically significant reduction of circulating levels of SPARC in NSCLC patients subjected to pre-operative chemotherapy, suggesting that SPARC potentially represents an indicator of treatment response. However, this observation was restricted only to a limited number of NSCLC cases and needs further confirmation.
Despite the introduction of innovative targeted therapies and the recent advances in the characterization of new circulating biomarkers, the majority of lung cancer patients are diagnosed at advanced stages and their prognosis remains extremely poor. Therefore, there is still an urgent need to identify biomarkers for the early diagnosis of lung cancer.
The identification of novel blood-based protein markers represents a particularly attractive field of cancer research for the easy applicability in clinical practice. First of all, venous blood sampling allows patients screening and monitoring over time with minimally-invasive procedures; secondly, the availability of commercial assays for the measurement of circulating proteins levels guarantees the standardization of the techniques and the low costs of analysis. Among the proteins that can function as circulating biomarkers in cancer patients, the identification of ECM associated factors represents a new challenging opportunity. Actually, large genomic, transcriptomic and proteomic approaches have improved in the last years the knowledge of the cancer matrisome. The matrisome has been defined as the whole of genes coding both for structural ECM proteins (collagens, laminins, fibrillins, tenascins, proteoglycans, etc.) and for ECM regulating proteins (metalloproteinases, metalloproteases, cathepsins, annexins, etc.), and it accounts for about 4% of the human genome and a total of 1,000 genes (14). Nevertheless, a complete characterization of all the ECM-related factors that enter into circulation and can be theoretically measured in liquid biopsies is still missing.
Several circulating ECM remodeling factors have been proposed as biomarkers for lung cancer diagnosis at early stage. For example, elevated serum levels of metalloprotease ADAM8 and of the fragments of both type I collagen (C1M) and citrullinated vimentin (VICM) have been correlated to the diagnosis of NSCLC (15,16). Differently, soluble syndecan-1 and metalloproteinase MMP-1 have been proposed as prognostic biomarkers, since high circulating levels of both proteins have been associated to poor prognosis of lung cancer patients (17,18).
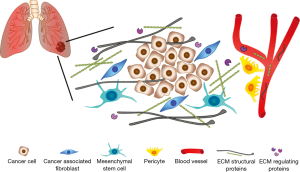
A limitation of the work of Andriani and coauthors is that they restricted their observations only on a small number of genes of the ECM3 panel, and did not analyze any of the other differentially expressed genes included in the ECM deposition and remodeling pathways significantly enriched in CAFs. In this regard, the analysis of combinations of different circulating ECM proteins could significantly improve the specificity and the sensibility of a potential diagnostic/prognostic test for the screening of NSCLC patients. In the paper by Giussani (4), for example, the authors demonstrated that the combination of the circulating levels of three different ECM molecules—COL11A1, COL10A1 and cartilage oligomeric matrix protein (COMP)—was able to better discriminate between breast cancer patients and controls compared to the analysis of single proteins. Moreover, Lim and coauthors have recently identified an ECM signature composed of 29 genes with both prognostic and predictive value for NSCLC patients, through a meta-analysis of ten independent publicly available microarray data sets comparing early stage NSCLC tumors and normal lung tissue samples. Such signature allows the stratification of NSCLC patients into low- and high-risk groups, according to overall survival and relapse-free survival rates, and can be used also as a predictive indicator of adjuvant chemotherapy response (19). Interestingly, COL11A1 and COL10A1 were included in the list of 29 genes of the signature, and the high-risk group of NSCLC patients displayed elevated intratumoral expression of both factors. In addition, though CAFs represent the major source of ECM proteins, other cell types of the lung tumor microenvironment, such as pericytes and mesenchymal stem cells, and cancer cells themselves, are involved in ECM remodeling, thus contributing to the circulating levels of ECM turnover products (Figure 1). Therefore, it is probable that other ECM proteins, albeit not differentially expressed between CAFs and NFs, have the requirements to be eligible as biomarkers for lung cancer, i.e., early induction during tumorigenesis and absent/low expression in healthy subjects.
It should be also considered that several pathological conditions regulate the expression of ECM proteins, such as arthritis, cardiomyopathies, cystic fibrosis and different lung diseases, including asthma, idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease (20). These disorders may affect the specificity of hypothetical diagnostic tests based on the analysis of circulating ECM protein levels, potentially representing an important cause of misleading results.
Finally, it must be emphasized that a number of different circulating biomarkers has been proposed for early detection of lung cancer, including miRNAs, circulating tumor cells (CTCs) and cell free DNA (cfDNA) (21,22). Intriguingly, the combination of cfDNA and circulating proteins seems particularly promising in terms of sensitivity and specificity for the detection of early cancer (23). In fact, the use of protein biomarkers can reduce the possible false negative results obtained with the analysis of cfDNA (24,25). In this respect, it might be useful in the future to combine the measurement of circulating ECM proteins with other biomarkers associated with lung tumorigenesis to improve the diagnostic ability of a potential screening test.
In summary, ECM molecules regulate all the hallmarks of cancer, from initiation to metastatic progression, and are actively released into circulation during tumorigenesis. For these reasons, they represent an intriguing source of new potential biomarkers for cancer diagnosis and prognosis. Among the ECM proteins identified in the study by Andriani and colleagues, SPARC seems to be a promising candidate biomarker for the screening of NSCLC patients. However, the existing data must be interpreted with caution and the use of circulating levels of ECM proteins for the diagnosis and possibly prognosis of NSCLC needs further validation in larger prospective studies before translation in clinical practice.
Acknowledgements
Funding: Dr. Normanno is supported by a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC) (grant number: IG17135).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012;196:395-406. [Crossref] [PubMed]
- Leeming DJ, Bay-Jensen AC, Vassiliadis E, et al. Post-translational modifications of the extracellular matrix are key events in cancer progression: opportunities for biochemical marker development. Biomarkers 2011;16:193-205. [Crossref] [PubMed]
- Andriani F, Landoni E, Mensah M, et al. Diagnostic role of circulating extracellular matrix-related proteins in non-small cell lung cancer. BMC Cancer 2018;18:899. [Crossref] [PubMed]
- Giussani M, Landoni E, Merlino G, et al. Extracellular matrix proteins as diagnostic markers of breast carcinoma. J Cell Physiol 2018;233:6280-90. [Crossref] [PubMed]
- Chong IW, Chang MY, Chang HC, et al. Great potential of a panel of multiple hMTH1, SPD, ITGA11 and COL11A1 markers for diagnosis of patients with non-small cell lung cancer. Oncol Rep 2006;16:981-8. [PubMed]
- Sole X, Crous-Bou M, Cordero D, et al. Discovery and validation of new potential biomarkers for early detection of colon cancer. PLoS One 2014;9:e106748. [Crossref] [PubMed]
- Chapman KB, Prendes MJ, Sternberg H, et al. COL10A1 expression is elevated in diverse solid tumor types and is associated with tumor vasculature. Future Oncol 2012;8:1031-40. [Crossref] [PubMed]
- Arnold SA, Brekken RA. SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signal 2009;3:255-73. [Crossref] [PubMed]
- Papapanagiotou A, Sgourakis G, Karkoulias K, et al. Osteonectin as a screening marker for pancreatic cancer: A prospective study. J Int Med Res 2018;46:2769-79. [Crossref] [PubMed]
- Ikuta Y, Nakatsura T, Kageshita T, et al. Highly sensitive detection of melanoma at an early stage based on the increased serum secreted protein acidic and rich in cysteine and glypican-3 levels. Clin Cancer Res 2005;11:8079-88. [Crossref] [PubMed]
- Zhang J, Hao N, Liu W, et al. In-depth proteomic analysis of tissue interstitial fluid for hepatocellular carcinoma serum biomarker discovery. Br J Cancer 2017;117:1676-84. [Crossref] [PubMed]
- Koukourakis MI, Giatromanolaki A, Brekken RA, et al. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res 2003;63:5376-80. [PubMed]
- Huang Y, Zhang J, Zhao YY, et al. SPARC expression and prognostic value in non-small cell lung cancer. Chin J Cancer 2012;31:541-8. [PubMed]
- Socovich AM, Naba A. The cancer matrisome: From comprehensive characterization to biomarker discovery. Semin Cell Dev Biol 2019;89:157-66. [Crossref] [PubMed]
- Ishikawa N, Daigo Y, Yasui W, et al. ADAM8 as a novel serological and histochemical marker for lung cancer. Clin Cancer Res 2004;10:8363-70. [Crossref] [PubMed]
- Willumsen N, Bager CL, Leeming DJ, et al. Serum biomarkers reflecting specific tumor tissue remodeling processes are valuable diagnostic tools for lung cancer. Cancer Med 2014;3:1136-45. [Crossref] [PubMed]
- Joensuu H, Anttonen A, Eriksson M, et al. Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Res 2002;62:5210-7. [PubMed]
- Li M, Xiao T, Zhang Y, et al. Prognostic significance of matrix metalloproteinase-1 levels in peripheral plasma and tumour tissues of lung cancer patients. Lung Cancer 2010;69:341-7. [Crossref] [PubMed]
- Lim SB, Tan SJ, Lim WT, et al. An extracellular matrix-related prognostic and predictive indicator for early-stage non-small cell lung cancer. Nat Commun 2017;8:1734. [Crossref] [PubMed]
- Gu BH, Madison MC, Corry D, et al. Matrix remodeling in chronic lung diseases. Matrix Biol 2018;73:52-63. [Crossref] [PubMed]
- Seijo LM, Peled N, Ajona D, et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J Thorac Oncol 2019;14:343-57. [Crossref] [PubMed]
- Gallo M, De Luca A, Maiello MR, et al. Clinical utility of circulating tumor cells in patients with non-small-cell lung cancer. Transl Lung Cancer Res 2017;6:486-98. [Crossref] [PubMed]
- Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018;359:926-30. [Crossref] [PubMed]
- Pasquale R, Bergantino F, Fenizia F, et al. Analysis of cfDNA reveals high levels of tumor heterogeneity in NSCLC. 60th Annual Meeting of the Italian Cancer Society. Available online: https://www.cancerologia.it/assets/sic2018-final-program.pdf
- Hu Y, Ulrich BC, Supplee J, et al. False-Positive Plasma Genotyping Due to Clonal Hematopoiesis. Clin Cancer Res 2018;24:4437-43. [Crossref] [PubMed]


