
Thymic epithelial tumor complicated by immunological abnormalities: results from a single-center retrospective study in China
Introduction
The thymus is a primary lymphatic organ responsible for several immune functions, which include generating a diverse repertoire of functional T cells. Through positive and negative selection of T cells, the thymus plays a role in immune self-tolerance to prevent autoimmunity. Furthermore, the thymus can secrete hormones and soluble factors such as thymosin (1). Thymic epithelial tumors (TETs) are rare tumors originating from epithelial cells of the thymus. The World Health Organization (WHO) subdivides TETs into types A, AB, B1, B2, and B3 (and additional rare types) thymomas and thymic carcinomas (TCs) (2,3).
Although the exact mechanisms have not been elucidated, there is an association between TET and immunological abnormalities, including autoimmune disease (AD) and immunodeficiency. Immunodeficiency, which is known as good syndrome (GS), is a complication of thymoma, with an incidence of 0.2–6% (4). In addition, a connection between thymoma and myasthenia gravis (MG) has been well documented (5). Co-occurrence of other ADs, such as systemic lupus erythematosus (6) and pure red cell aplasia (PRCA) (7), has been described in case reports. TCs are approximately 10 times less prevalent than thymoma, which are more aggressive and refractory to treatment; however, they are reported to relate to ADs in previous studies (8,9).
Clinical data on GS and AD cases with comorbid TET have rarely been reported in China. To improve our knowledge on immunological abnormalities in this rare disease, we retrospectively analyzed clinical data from patients with histologically confirmed TET over a five-year period at our institution.
Methods
We retrospectively reviewed clinical information from a group of 59 patients with TET at ChaoYang Hospital between January 2013 and May 2018.
TET diagnosis was made based on clinical manifestations, chest high-resolution computed tomography, and pathology results. Pathologic type was determined according to the 2004 WHO histological classification. GS was defined as comorbid thymoma and hypogammaglobulinemia. ADs were diagnosed based on classification diagnostic criteria for rheumatoid arthritis (RA), MG, and Sjogren’s syndrome (SS).
Clinical characteristics were acquired through a review of patient medical records, and included age, gender, WHO type, clinical signs and manifestations, laboratory parameters (immunological features), treatment procedures, and prognosis.
Quantitative data were expressed as the median (range) or mean ± standard deviation, and qualitative data were expressed as percentages. Statistical analyses were performed using SPSS 17.0 software (Chicago, IL, USA). Categorical variables were compared using the χ2 test or Fisher’s exact test, while continuous variables were compared using the Student’s t-test or Mann-Whitney U test. A two-sided P value <0.05 was considered statistically significant.
Results
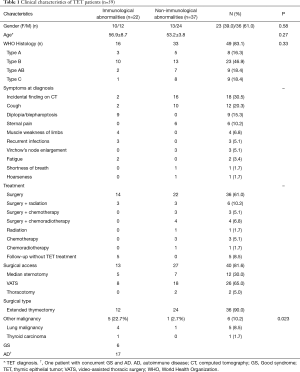
Table 1 shows clinical characteristics of TET patients in this study. TET diagnosis was made based on incidental findings from chest high-resolution computed tomography in 30.5% of patients (n=18). The following symptoms were present at the time of diagnosis: cough (n=12), diplopia and/or blepharoptosis (n=9), sternal pain (n=6), muscle weakness of limbs (n=4), recurrent infections (n=3), Virchow’s node enlargement (n=3), fatigue (n=2), shortness of breath (n=1), and hoarseness (n=1).
Full table
Histological TET type data were available for 49 (83.1%) patients. The most common type was B (46.9%), followed by AB, C, and A, with frequencies of 18.4%, 18.4%, and 16.3%, respectively.
Forty-nine patients underwent thymectomy (40 patients had definite surgical information; Table 1), of whom six patients received radiation and three patients received chemotherapy following surgery. Four patients were administered chemoradiotherapy following surgery. Five patients remained under follow-up without a specific TET treatment.
Twenty-two (37.3%) out of the 59 patients had comorbid immunological abnormalities. Six patients (10.2%) developed a second malignancy during follow up. There were no significant differences between groups across gender (P=0.58), age (56.9±8.7 vs. 53.2±3.8 years; P=0.27), or histological type (P=0.33), regardless of immunological abnormalities; however, there was a significant difference in developing a second malignancy (22.7% vs. 2.7%; P=0.023), and the percentage was higher in TET patients complicated by immunological abnormalities (Table 1).
Clinical features of GS
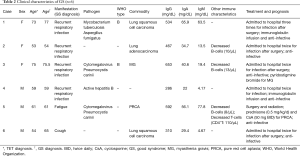
Table 2 shows clinical characteristics of the GS patients. Six patients (10.2%) met the criteria for GS, with a 1:1 male to female ratio. The average age of TET diagnosis was 62.5 years (range, 53–75 years), while the average age of GS diagnosis was 65.3 years (range, 54–77 years). Four cases were diagnosed after thymectomy, while the other cases were identified at the same time of TET diagnosis. Two patients had histologically confirmed type B.
Full table
All patients presented with marked hypogammaglobulinemia with normal lymphocyte levels. Three patients were tested for lymphocytes subsets, and all of them presented with reduced circulating B cells; CD4+T cells were reduced in one patient.
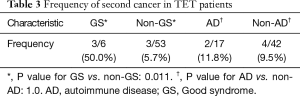
The major manifestations of this condition were recurrent respiratory infections, and five of six patients had hospital admissions due to infection more than once. Pathogens were identified in three patients, including cytomegalovirus and pneumocystis carinii in two patients and mycobacterium tuberculosis and aspergillus fumigatus in one patient. One patient presented with both active hepatitis B and bronchiectasis; one patient had PRCA after two months of thymectomy; one patient had MG before GS diagnosis. Three GS patients developed another malignancy (lung cancer), which was significantly higher than patients with TET without GS (50% vs. 5.7%; P=0.011) (Table 3).
Full table
In the five patients that underwent surgery, infections were not resolved. Two patients received immunoglobulin supplementation at the time of infection. The patient with PRCA was administered cyclosporine and steroids without prophylactic antibiotics and hemoglobin responded well; however, during follow up, the patient acquired an opportunistic infection.
Clinical features of ADs
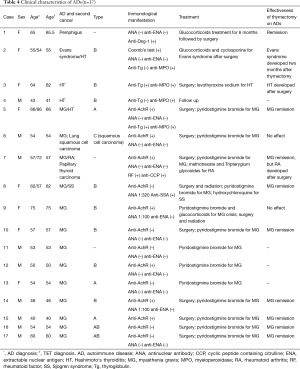
Table 3 shows clinical characteristics of ADs patients. Seventeen (28.8%) in 59 cases presented with ADs, with a male to female ratio of 9:8. The average age of TET diagnosis was 56 years (range, 40–75 years). MG (n=13) was the most common AD, followed by Hashimoto’s thyroiditis (HT) (n=4), SS (n=1), RA (n=1), pemphigus (n=1), and Evans syndrome (n=1). Four patients developed two ADs (MG and RA; MG and SS; MG and HT; Evans syndrome and HT). Thirteen patients were diagnosed with TET due to MG diagnosis, while three AD conditions (pemphigus, HT, and SS) developed before TET diagnosis; however, three AD conditions (RA, HT, and Evans syndrome) occurred after thymectomy, ranging from two months to 15 years. Two patients developed a second cancer; however, the frequency was not significantly higher compared to TET patients without ADs (11.8% vs. 9.5%; P=1.0) (Table 3).
Among the 17 ADs patients, histological classification data were available for 14 (82.4%). The most common type was B (57.2%), while A, AB, and C types were found in 21.4%, 14.3%, and 7.1% of patients, respectively. The frequency of ADs (P=0.59) and MG (P=0.64) was not significantly different across the histological classification groups.
Thirteen patients (including 10 MG patients) underwent thymectomy, and two of these patients received radiation therapy. All 13 MG patients were administered pyridostigmine bromide and responded well to treatment. One case of MG crisis was treated with glucocorticoids. Following thymectomy, 8 MG patients and 1 pemphigus patient stopped oral medicine therapy without experiencing AD relapses. Other treatments are shown in Table 4.
Full table
Discussion
As an important immune organ, it is rational to speculate that tumors arising from thymus epithelial cells can result in immune dysfunction. In the present study, approximately 40% of thymoma patients presented with comorbid immunological abnormalities, consistent with previous findings (10). However, these data were lower than what has been reported in other studies (8,11).
Here, we identified several interesting findings as follows: (I) there is an association between immunological abnormalities and TET (many patients presented with MG first); (II) immunological abnormalities can occur at any time during the disease, even after thymectomy; (III) in addition to opportunistic infection, GS patients are prone to develop another malignancy; (IV) thymectomy may produce favorable outcomes for MG patients but it is not effective for GS patients with recurrent infection; (V) some ADs, such as RA and Evans syndrome, should be treated with immunosuppressants.
In 1954, Robert Good described three patients with thymoma and hypogammaglobulinemia, which is known today as GS. The principal immunological feature of GS is hypogammaglobulinemia, with a reduction in peripheral B-cells and/or CD4+ T-cells and/or a reversed ratio of CD4 to CD8 T cells (12). While leukopenia is considered as an immune characteristic of GS patients, we did not identify this phenomenon in our study (13). The pathogenesis of GS remains elusive, although there is evidence that T cells from patients with thymoma can inhibit immunoglobulin production via B (14) and pre-B cell growth (15).
Kelesidis et al. [2010] reported that patients with GS usually present in the 4th or 5th decade of life (4). However, we found that patients were diagnosed with GS at an older age, indicating a potential lack of awareness of this disease. Similarly to previous reports, we found that male and female patients were equally affected by GS (13). Additionally, immunodeficiency may precede or occur after thymoma diagnosis, even after thymectomy (16,17). Although MG is a common comorbidity of thymoma, Malphettes et al. [2015] reported that it is relatively rare in GS patients (18); however, one of the GS patients in this study did indeed present with MG.
The main clinical manifestation of GS is susceptibility to infections of encapsulated bacteria, viruses, and fungi in the absence of immunosuppressive treatment. Severe infection, usually in the respiratory and intestinal tracts, is the major cause of morbidity and mortality (13,19). In contrast to common variable immune deficiency, opportunistic infections have been found to commonly occur with dysfunction of cell-mediated immunity in GS (12). The Respiratory and Infectious Disease Departments in our hospital are well known, and, therefore, there are a greater number of patients with recurrent infections, possibly explaining why the frequency of GS in this study was higher than what has previously been reported.
Gadalla et al. [2011] reported that thymoma patients have a two-fold risk of developing a second cancer compared with the general population (10). We found that the risk of a second cancer was higher in GS patients. This observation might be explained by immunodeficiency and/or immune system alteration in association with thymectomy. Thymectomy is recommended in TET patients to prevent locally invasive growth and metastasis of tumor cells; however, it may not improve immunodeficiency in GS, which is in line with previous studies (4). Immunoglobulin intravenous infusion and anti-infective treatment are recommended for this condition.
Risk of comorbid thymoma and ADs is increased during the 5th and 6th decades of life, which is agreement with this study (20). MG is the most common AD linked to TET. A previous study found that 75% of patients with MG developed a thymus abnormality, including thymic hyperplasia in 85% of patients and thymoma in 15% of cases (21). Approximately 30% of thymoma patients developed MG (22). In our study, 22% (13/59) of TET patients had comorbid MG. To date, the relationship between pathological type and ADs remains unknown. Okumura et al. [2008] found that MG is frequently associated with WHO type B thymoma (23), and Zhang et al. [2016] reported that the prevalence of MG is higher in type B2 thymoma compared with other histological types (24). In this study, we did not find an association between histological type and MG. Thymectomy is recommended in thymoma-associated MG regardless of clinical symptoms, while pretreatment with immunosuppressive drugs may be necessary to prevent perioperative deterioration (25). In the present study, 77% (10/13) of MG patients underwent resection and all 13 patients received oral drugs. Following thymectomy, eight patients (80%; 8/10) stopped therapy without experiencing a MG relapse. Three patients who only received oral treatment responded well but their cancer did not regress.
PRCA is an anemic condition characterized by the absence of reticulocytes in the blood and erythroid precursors in the bone marrow, while other hematopoietic cell lineages are present with no morphological abnormalities. PRCA is secondary to various diseases, including large-cell granular lymphocyte leukemia, solid tumors, and viral infections. Approximately 10% of PRCA cases are associated with thymoma (7), which can occur at any time during the disease duration (26). Kelesidis et al. [2010] reported that PRCA is the most common autoimmune complication associated with GS (4), and Okui et al. [2017] found that type AB is the most common histological type in cases of GS complicated by PRCA (27). Although thymectomy is effective for some thymoma-associated PRCA cases (28), PRCA can reoccur after thymoma removal (26). Cyclosporine has been used for the treatment of thymoma-associated PRCA with remarkable efficacy (26,27), which even been shown to reduce tumor size (29,30). In our study, thymoma-associated PRCA and GS were found simultaneously after thymectomy and anemia responded well to cyclosporine with corticosteroids. However, opportunistic infections after immunosuppressive treatment must be carefully monitored.
Other co-morbid hematological disorders have been reported, such as immune thrombocytopenia, autoimmune hemolytic anemia, and thrombotic thrombocytopenia purpura, but not Evans syndrome (8). In this study, one patient presented with Evans syndrome after thymectomy and responded well to corticosteroids and cyclosporine. In a previous study, 82% of thymoma patients with autoimmune hemolytic anemia were treated with corticosteroids and 78% of patients achieved clinical remission (31).
Paraneoplastic pemphigus is a group of life-threatening autoimmune blistering skin disorders that can occur in cancer patients. Thymectomy can decrease antibody titer and improve clinical outcomes in thymoma-related pemphigus (32,33). In this study, one pemphigus patient who underwent surgery after six months of steroid treatment did not experience a disease flare.
Cases of HT have also been described in thymoma (34,35). Epidemiological studies have shown that thyroid diseases occur in approximately 5–11.9% of MG patients (36). HT was the second most common AD in this study. We also identified cases of RA and SS, which are relatively rarely reported in thymoma patients (35,37). These conditions may be treated with oral medicines following surgery. The relationship between these ADs and TET still needs more investigation.
There is a clear association between TET and immunological abnormalities, including AD and immunodeficiency. These cases may occur before or after a diagnosis of TET and can even occur after thymectomy. When TET is diagnosed, serum immunoglobulin should be evaluated to identify GS as soon as possible due to its poor prognosis. Since opportunistic infections are common in GS, diagnosis may provide direction for empirical antibiotic therapy before acquiring pathogen information. As identified in this study, patients should be monitored for occurrence of a second malignancy. If TET patients present with clinical manifestation indicative of ADs, autoantibody tests should be conducted.
There were some limitations in our study. First, data were collected in a retrospective manner and some data points were missing; in addition, follow-up data, including prognosis and relapse rates, were also missing for some patients. Since some pathological outcomes were provided by other institutions, WHO type information was incomplete. Second, the study sample was small, thus limiting the power of the analysis. Third, we cannot provide risk factors for TET with immunological abnormalities based on the analyses performed.
Conclusions
There is a strong association between TET and immunological abnormalities, including AD and immunodeficiency. These cases may occur at any disease duration, even after thymectomy. In addition to opportunistic infections, risk of a second cancer is higher in GS patients. Thymectomy may have favorable outcomes for some ADs, especially MG, but does not improve immunodeficiency in GS patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As this is a retrospective study of existing clinical data analysis, no human samples were re-acquired or retested; therefore, we did not apply for ethical approval from our Institutional Review Board. However, all patients signed a written informed consent allowing publication of this manuscript.
References
- Miller JF. Revisiting thymus function. Front Immunol 2014;5:411. [Crossref] [PubMed]
- Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg 2004;77:1183-8. [Crossref] [PubMed]
- Marx A, Strobel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. [Crossref] [PubMed]
- Kelesidis T, Yang O. Good's syndrome remains a mystery after 55 years: A systematic review of the scientific evidence. Clin Immunol 2010;135:347-63. [Crossref] [PubMed]
- Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016;87:419-25. [Crossref] [PubMed]
- Bozzolo E, Bellone M, Quaroni N, et al. Thymoma associated with systemic lupus erythematosus and immunologic abnormalities. Lupus 2000;9:151-4. [Crossref] [PubMed]
- Thompson CA, Steensma DP. Pure red cell aplasia associated with thymoma: clinical insights from a 50-year single-institution experience. Br J Haematol 2006;135:405-7. [Crossref] [PubMed]
- Bernard C, Frih H, Pasquet F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 2016;15:82-92. [Crossref] [PubMed]
- Katzberg HD, Miller RG, Katz J. Thymic carcinoma in myasthenia gravis developing years after thymectomy. Muscle Nerve 2009;40:137-8. [Crossref] [PubMed]
- Gadalla SM, Rajan A, Pfeiffer R, et al. A population-based assessment of mortality and morbidity patterns among patients with thymoma. Int J Cancer 2011;128:2688-94. [Crossref] [PubMed]
- Holbro A, Jauch A, Lardinois D, et al. High prevalence of infections and autoimmunity in patients with thymoma. Hum Immunol 2012;73:287-90. [Crossref] [PubMed]
- Kelleher P, Misbah SA. What is Good's syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol 2003;56:12-6. [Crossref] [PubMed]
- Sun X, Shi J, Wang M, et al. Good's Syndrome Patients Hospitalized for Infections: A Single-Center Retrospective Study. Medicine (Baltimore) 2015;94:e2090. [Crossref] [PubMed]
- Litwin SD, Zanjani ED. Lymphocytes suppressing both immunoglobulin production and erythroid differentiation in hypogammaglobulinaemia. Nature 1977;266:57-8. [Crossref] [PubMed]
- Hayward AR, Paolucci P, Webster AD, et al. Pre-B cell suppression by thymoma patient lymphocytes. Clin Exp Immunol 1982;48:437-42. [PubMed]
- Raschal S, Siegel JN, Huml J, et al. Hypogammaglobulinemia and anemia 18 years after thymoma resection. J Allergy Clin Immunol 1997;100:846-8. [Crossref] [PubMed]
- Qu J, Lu X, Gao Q, et al. Good Syndrome, a rare cause of refractory chronic diarrhea and recurrent pneumonia in a Chinese patient after thymectomy. Clin Vaccine Immunol 2013;20:1097-8. [Crossref] [PubMed]
- Malphettes M, Gerard L, Galicier L, et al. Good syndrome: an adult-onset immunodeficiency remarkable for its high incidence of invasive infections and autoimmune complications. Clin Infect Dis 2015;61:e13-9. [Crossref] [PubMed]
- Jansen A, van Deuren M, Miller J, et al. Prognosis of Good syndrome: mortality and morbidity of thymoma associated immunodeficiency in perspective. Clin Immunol 2016;171:12-7. [Crossref] [PubMed]
- Aarli JA. Myasthenia gravis in the elderly: Is it different? Ann N Y Acad Sci 2008;1132:238-43. [Crossref] [PubMed]
- Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve 2008;37:141-9. [Crossref] [PubMed]
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015;14:1023-36. [Crossref] [PubMed]
- Okumura M, Fujii Y, Shiono H, et al. Immunological function of thymoma and pathogenesis of paraneoplastic myasthenia gravis. Gen Thorac Cardiovasc Surg 2008;56:143-50. [Crossref] [PubMed]
- Zhang Z, Cui Y, Jia R, et al. Myasthenia gravis in patients with thymoma affects survival rate following extended thymectomy. Oncol Lett 2016;11:4177-82. [Crossref] [PubMed]
- Gilhus NE, Skeie GO, Romi F, et al. Myasthenia gravis - autoantibody characteristics and their implications for therapy. Nat Rev Neurol 2016;12:259-68. [Crossref] [PubMed]
- Hirokawa M, Sawada K, Fujishima N, et al. Long-term response and outcome following immunosuppressive therapy in thymoma-associated pure red cell aplasia: a nationwide cohort study in Japan by the PRCA collaborative study group. Haematologica 2008;93:27-33. [Crossref] [PubMed]
- Okui M, Yamamichi T, Asakawa A, et al. Pure Red Cell Aplasia Associated with Good Syndrome. Korean J Thorac Cardiovasc Surg 2017;50:119-22. [Crossref] [PubMed]
- Zeok JV, Todd EP, Dillon M, et al. The role of thymectomy in red cell aplasia. Ann Thorac Surg 1979;28:257-60. [Crossref] [PubMed]
- Mochizuki H, Okada T, Yoshizawa H, et al. Cyclosporin improved pure red cell aplasia associated with thymoma and tended to decrease thymoma size: a case report. Nihon Kokyuki Gakkai Zasshi 2003;41:755-9. [PubMed]
- Isshiki Y, Tanaka H, Suzuki Y, et al. Cyclosporine is a potential curative treatment option for advanced thymoma. Exp Hematol Oncol 2017;6:13. [Crossref] [PubMed]
- De Keyzer K, Peeters P, Verhelst C, et al. Autoimmune haemolytic anaemia associated with a thymoma: case report and review of the literature. Acta Clin Belg 2009;64:447-51. [Crossref] [PubMed]
- Lim JM, Lee SE, Seo J, et al. Paraneoplastic Pemphigus Associated with a Malignant Thymoma: A Case of Persistent and Refractory Oral Ulcerations Following Thymectomy. Ann Dermatol 2017;29:219-22. [Crossref] [PubMed]
- Yoshida M, Miyoshi T, Sakiyama S, et al. Pemphigus with thymoma improved by thymectomy: report of a case. Surg Today 2013;43:806-8. [Crossref] [PubMed]
- Yapali S, Oruc N, Ilgun S, et al. Acute presentation of autoimmune hepatitis in a patient with myasthenia gravis, thymoma, Hashimoto thyroiditis and connective tissue disorder. Hepatol Res 2012;42:835-9. [Crossref] [PubMed]
- Zou L, Xiong Z, Dun Y, et al. A case of rheumatoid arthritis associated with Hashimoto's thyroiditis and thymoma. Int J Rheum Dis 2017;20:1792-3. [Crossref] [PubMed]
- Kanazawa M, Shimohata T, Tanaka K, et al. Clinical features of patients with myasthenia gravis associated with autoimmune diseases. Eur J Neurol 2007;14:1403-4. [Crossref] [PubMed]
- Tsai Y, Lin Y, Chen C, et al. Thymoma associated with myasthenia gravis and Sjogren syndrome. West Indian Med J 2013;62:264-5. [PubMed]


