Clinical outcome and hemodynamic performance of St. Jude Trifecta aortic prosthesis: short-term follow-up and risk factors analysis
Introduction
The Trifecta aortic prosthesis (St. Jude Medical, Inc., St. Paul, MN, USA) is a tri-leaflet stented pericardial biological prosthetic valve designed for the aortic supra-annular aortic valve replacement. The bovine pericardial sheet is mounted outside the stent frame, which allows for almost circular cross-section during systole. Several reports have demonstrated favourable hemodynamic profile, i.e. low peak and mean trans-prosthetic gradients, excellent effective orifice area (EOA), low incidence of patient-prosthesis mismatch (PPM) also in patients with a small aortic annulus (1-4). Moreover, the excellent fluid dynamic characteristics of the Trifecta aortic prosthesis have been favourably compared with those reported for the stentless biological valves (5,6). However, given the fact that the Trifecta aortic valve has been introduced quite recently, follow-up data are not well still investigated, especially concerning the structural valve deterioration, the event-free valve-related survival, and the hemodynamic performance of the valve over time. The present study aims to evaluate clinical outcome and hemodynamic performance of the third-generation biological aortic valve prosthesis Trifecta up to 3 years of follow-up.
Methods
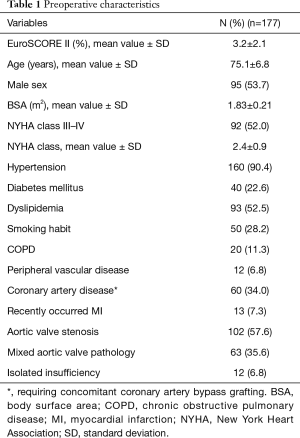
Between December 2014 and December 2017, at the Cardiac Surgery Division of the Tor Vergata University Hospital 177 patients (95 males, 82 females; mean age 75.1±6.8 years) received a St. Jude Trifecta aortic prosthesis were followed for a 3-year period and represented the object of our investigation. Preoperative mean value of NYHA class was 2.4±0.9. Ninety-two patients (52.0%) were in NYHA class III–IV. The EuroSCORE II was 3.2%±2.1%. Preoperative clinical characteristics have been reported in Table 1. Inclusion criteria of the study considered all patients who underwent primary aortic valve replacement as isolated procedure (n=117) or in combination with coronary artery bypass grafting (n=60), electively (n=112) or in an urgent setting (n=65). The major indications for aortic valve replacement were aortic valve stenosis (n=102) (57.6%), steno-insufficiency (n=63) (35.6%), pure insufficiency (n=12) (6.8%) (Table 1). The average preoperative value of the EOA was 0.48±0.18 cm2.
Full table
The study was approved by the Institutional Review Board of the Tor Vergata University Hospital, which waived the need for patient consent. All patients gave informed surgical consent. The study was designed to be as retrospective one.
Surgical technique
Surgery was performed through a median sternotomy or a mini-sternotomy approach with a “J” incision. Once cardiopulmonary bypass was started, after cross-clamping the ascending aorta and performing the cardiac arrest using warm blood cardioplegia or St. Thomas cold crystalloid solution, the aorta was opened with a transverse aortotomy, 1.0–1.5 cm distally to the origin of the right coronary artery and extended circumferentially. Excision of the cusps was started with scissors into the right cusp between the right coronary ostium and the commissure between the right coronary and non-coronary cusps; the calcific deposits were taken away from the aortic annulus with a small surgical spatula. After the excision of the cusps, supra-annular Trifecta aortic valve implantation was performed using 10–16 double-needled 2-0 synthetic sutures, accordingly with the size of the prosthesis, using teflon pledgets on sub-annular position. After Trifecta prosthesis placement, the aortotomy was closed with a 4-0 polypropylene double continuous suture. Concomitant coronary artery bypass grafting was performed with the use of the left internal thoracic artery to graft the left anterior descending artery and saphenous vein single grafts for right coronary artery and circumflex artery territories. Trans-oesophageal echocardiography was performed intraoperatively and at weaning from cardiopulmonary bypass in all patients.
Definitions, data collection
Operative mortality included death in hospital after operation at anytime, or within 30 days after discharge. The follow-up was performed by clinical evaluation and trans-thoracic echocardiograms, at 26.5±9.1 (median 31) months after operation. Where the follow-up was not possible (dead patients), medical data were collected by telephone interview of family members and or confirmed by physicians. Adverse events were classified according to the standardized definitions from the Society of Thoracic Surgeons and the American Association for Thoracic Surgery “Guidelines for reporting morbidity and mortality and cardiac valve interventions” (7). Follow-up was closed on 30th April 2018, and was 100% complete. Trans-thoracic echocardiographic data were recorded preoperatively, at discharge and during follow-up. Standard prosthetic valve measurements were obtained according to the criteria of the American Society of Echocardiography (8). Peak and mean aortic valve gradients, EOA, EOA index (EOAI), left ventricular ejection fraction, end-diastolic diameter and volume, end-systolic diameter and volume, septum and posterior wall thickness, pulmonary arterial pressure were recorded. Aortic valve regurgitation was classified as none (0/4+), trivial (1+/4), mild to moderate (2+/4), moderate to severe (3+/4) and severe (4+/4), according with the width of the regurgitation jet compared to that of the outflow tract (9). PPM was defined as mild-to-moderate in presence of EOAI >0.80 cm2/m2 and ≤0.85 cm2/m2, moderate in presence of EOAI >0.60 cm2/m2 and ≤0.80, severe in presence of EOAI <0.60 cm2/m2 (10).
Statistical analysis
It was performed with Stat View 4.5 (Abacus Concepts, Berkeley, CA, USA). Univariate analysis of preoperative and perioperative variables considered as potential risk factors for operative mortality was performed using the Student’s t-test for continuous data and the Chi-Squared or Fisher’s exact test for categorical data. Univariate variables with a P value of or less than 0.1 were included in the multivariate Logistic Regression analysis. The following preoperative and perioperative variables were included in the univariate and the multivariate analyses. Preoperative variables were age, sex, EuroSCORE II (11), body surface area, body mass index, co-morbidity, i.e., arterial hypertension, diabetes mellitus, chronic obstructive pulmonary disease, peripheral vascular disease, chronic renal dysfunction, presence of chest pain evaluated by means of CCS class, NYHA class, left ventricular ejection fraction, end-systolic and end-diastolic diameters and volumes, septum and posterior wall thickness, aortic valve area, the aortic valve pathology, concomitant coronary artery disease, i.e. the presence of multiple vessel coronary artery disease and/or left main disease. Perioperative variables included the need for urgent surgery, cardiopulmonary bypass and aortic cross-clamp times, sizes of Trifecta prosthesis, presence of PPM, combined coronary artery bypass surgery, need for prolonged mechanical ventilation, the development of postoperative complications, i.e., acute kidney injury, neurological damage.
Overall survival (not including operative deaths), freedom from cardiac death, freedom form prosthetic valve-related events were expressed as mean values plus or minus one standard deviation, and computed by using the Kaplan-Meier method. The Mantel-Cox Log-rank test was used to compare survival estimates among subgroups, i.e., isolated aortic disease versus concomitant aortic valve and coronary disease. The Cox proportional hazards method was used to evaluate the influence of variables on time to death. All other values were expressed as mean plus or minus one standard deviation of the mean. The calculated echocardiographic parameters at the follow-up were compared with the preoperative ones. A P value less than 0.05 was considered statistically significant.
Results
Concomitant coronary artery bypass grafting was performed in 60 patients (34%); the prosthesis sizes of the Trifecta aortic valve were 19 mm in 46 patients (26%), 21 mm in 69 (39%), 23 mm in 46 (26%), 25 mm in 16 (9%). Cardiopulmonary bypass time was 86±29 and 122±37 minutes for isolated aortic valve replacement and with concomitant coronary artery surgery, aortic cross-clamp time 67±21 and 95±28 minutes, respectively.
Operative mortality was 3.4% (n=6). Causes of death included postoperative neurological damage (n=2), sudden death due to ventricular arrhythmia (n=1) and acute intracoronary thrombosis (n=1), respectively, low cardiac output syndrome followed by multi-organ failure (n=1), and sepsis (n=1). Operative mortality, as expected, was higher in the combined procedures in comparison with isolated aortic valve replacement (6.7% versus 1.7%), although this difference did not reach a statistical significance (P=0.084). The only independent predictor of operative mortality was the need for the mechanical ventilation greater than 24 hours (P=0.037). Recently occurring myocardial infarction was detected as a risk factor for higher mortality at the univariate analysis only (P=0.013).
Postoperatively, the incidence of permanent pacemaker implantation was 4.5% (n=8). Acute kidney injury occurred in four patients (2.3%), bleeding requiring surgical re-exploration in 10 (5.6%), neurological damage in 2 (1.1%). Both at the intraoperative transesophageal echocardiogram and at the transthoracic echocardiogram before discharge, no para-valvular aortic leaks have been shown.
Follow-up results
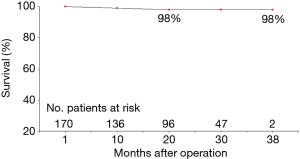
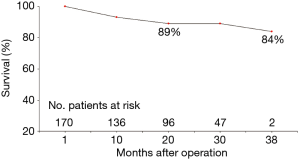
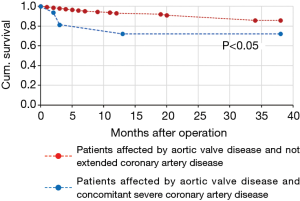
During follow-up, there were 18 deaths out of 171 patients (10.5%). Three patients died for cardiac causes (myocardial infarction in 1 patient, congestive heart failure in 1 patient, sudden death in another one), 3 for endocarditis complicated by septic shock, 10 for non-cardiac or non-prosthetic valve related causes, and 2 for unknown reasons. NYHA and CCS classes values significantly improved in comparison with the preoperative values (1.6±0.7 versus 2.4±0.9 and 0.1±0.4 versus 0.4±0.8, respectively; P<0.0001, for both comparisons), 92.1% of survived patients (n=141) were in NYHA class I–II. Three endocarditis occurred within six months from the operation and two of them required prosthesis explant, the other one after 8 months form the operation. No any case of acute valve thrombosis, thromboembolic event, haemolysis or structural valve deterioration was registered. Overall, 3-year actuarial survival was 84.0%±5.6% (Figure 1), freedom from cardiac death 98.0%±2.0% (Figure 2), freedom from prosthetic valve endocarditis 97.4%±1.3%. No independent predictors for reduced survival were recognized at the Cox Regression analysis. At the Mantel-Cox test the presence at the operation of a concomitant severe coronary artery disease, i.e., multi-vessel coronary artery and/or left main stem disease, did significantly affect the survival (72.0%±12.0% versus 86.0%±6.0%; P=0.015) (Figure 3).
Echocardiographic results
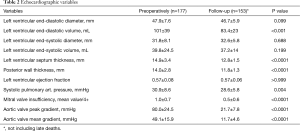
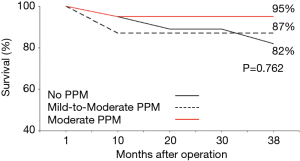
At discharge, mild-to-moderate PPM was present in 32 out of 171 alive patients (18.7%), moderate in 19 patients (11.1%), severe PPM was absent. As expected, the presence of PPM was more frequent after implantation of Trifecta aortic valve sizes 19 and 21 mm in comparison with 23 and 25 mm (P<0.0001). At 26.5±9.0 months of follow-up, mean and peak trans-aortic valve gradients were found significantly decreased, with a reduction to approximately more than 70% of the preoperative values (11.7±4.6 and 21.7±7.8 mmHg versus 49.1±15.9 and 80.0±24.5 mmHg, respectively; P<0.0001, for both comparisons). Echocardiographic variables measured preoperatively and at follow-up were reported and compared in Table 2. Average peak and mean gradients measured during follow-up were similar to those registered at discharge (11.8±4.6 versus 12.8±6.7 mmHg, and 21.7±7.8 versus 18.8±8.6 mmHg, respectively; P>0.1, for all comparisons). Mean and peak trans-aortic valve gradients were 12.9±5.0 and 22.8±7.2 mmHg for Trifecta prosthesis size 19 mm, 12.1±4.7 and 21.3±6.5 mmHg for size 21 mm, 11.0±4.0 and 20.6±7.3 mmHg for Trifecta prosthesis size 23 mm, 8.0±4.2 and 14.6±7.4 mmHg for Trifecta prosthesis size 25 mm. A peak gradient value equal or greater than 40 mmHg was documented in 3 cases out of 153 (2%) patients; on the contrary, a mean gradient greater than 30 mmHg was not found in any patient. The presence of PPM, in its mild-to-moderate or moderate degree, did not negatively affected late survival (Figure 4), nor lack of NYHA class improvement during the follow-up.
Full table
Discussion
Early and short-term outcomes
In our study we have showed a good safety profile of the valve with no valve-related perioperative complications and low operative mortality. In particular, as expected, operative mortality was higher in presence of concomitant coronary artery bypass surgery (6.7% versus 1.7%). In 100 patients (mean age 74.6±7.4 years) undergoing Trifecta aortic prosthesis implantation, Paredes and co-workers (12) reported 5% of operative mortality after aortic valve replacement in concomitance with other procedures. Tadokoro and colleagues (13) in 103 patients (mean age 72.9±8.3 years) with a mean 2.9% EuroSCORE II, reported 1% of operative mortality. Raimundo et al. (14) in 556 patients (mean age 73±9 years) with a mean 2.9% EuroSCORE II reported 5.4% of 30-day mortality; in 301 cases (54.1%) combined procedures were performed, especially concomitant coronary artery bypass grafting (30.6%). In our population, the incidence of coronary artery disease requiring concomitant coronary artery bypass grafting was similar (34%).
In a similar way to that we have observed in our study, Anselmi and colleagues (15) in a large series of 824 consecutive implants (mean age of patients 75.4±7.7 years) of Trifecta aortic prosthesis reported 3.8% of operative mortality, including 2.7% in patients undergoing isolated aortic valve replacement, 8.1% in those undergoing combined surgery.
The observed incidence of 4.5% of pacemaker implantation was similar to that reported by the above-mentioned studies (14,15) and by Lehmann and co-workers (16), ranging from 1.9% to 5.7%, and to that observed in our Institution after implantation of other third-generation biological prostheses. Finally, in a larger series of 1,801 patients undergoing isolated aortic valve replacement, Litwinowicz and collaborators (17) reported a short-term mortality rate of 0.3% (5 deaths).
For population of patients older than 70 years at the operation, the actuarial survival reported in the literature at three years after implantation of Trifecta prosthesis is estimated to be 83–88% (1,14,16): this reported rate was comparable to the survival rate reported in our investigation (84.0%±5.6%). In particular, we found that the concomitant severe coronary artery disease negatively affected the survival by about 14%. The reported incidences of freedom from valve-related death, from endocarditis, from thromboembolism, and from paravalvular leak appear satisfactory in the mid-term (2 up to 5 years) follow-up, being estimated 97–98%. Filip et al. (18) in a selected patient population aged 72±5.2 years at the time of operation undergone isolated aortic valve replacement with sutureless bioprostheses, reported a 100% survival rate a 5 years of follow-up. The 3-year freedom from cardiac death was 98.0%±2.0%, with three deaths for cardiac causes out of 18 deaths observed during follow-up.
Hemodynamic performance
The increasing need for biological prosthesis related to the rising age of the patients needing aortic valve replacement surgery, has stimulated the industries toward the research of the ideal prosthetic valve. The ideal biological prosthesis should allow the surgeon to use an easy, quick and safe implant technique with low risk for paravalvular leak or structural degeneration. The prosthesis should also have a low intrinsic thrombogenicity, and a high-quality hemodynamic performance with low gradients, large EOA, and good movement and coaptation of the leaflets. Trifecta aortic biological prosthesis is trying to address those requests with its features, and it has been designed with a concave, scalloped sewing ring for a supra-annular implant with non-everting sutures. Echocardiographic assessment of the hemodynamic performance of the Trifecta aortic prosthesis at three years revealed a satisfactory performance of this valve when compared with other pericardial prostheses (19-22). The nearly physiological hemodynamic performance of Trifecta prosthesis could decrease the need of stentless valves which, on the contrary, require a substantial learning curve, technically demanding implantation and an aortic root replacement in case of failure of the prosthesis (23). The external mounting of the leaflets allows for a wider opening, and the expansible stent could limit the impedance to flow during high flow conditions, i.e. during exercise (24). The nearly cylindrical opening of the Trifecta during systole provides gradients and EOAs that can result superior to any other available stented aortic prosthesis and similar to those observed for a stentless valve (5). Bavaria and colleagues (25) provided excellent hemodynamic performance of Trifecta prosthesis in more than 1,000 patients enrolled at 31 centers, documenting at the time of discharge an average mean gradients ranging from 9.3 to 4.1 mmHg. The present study confirmed this excellent hemodynamic performance with an average mean and peak gradients across all valve sizes of 12.8 and 18.8 mmHg, respectively, that remained stable at the follow-up echocardiographic evaluation. Indeed, the mean gradient at follow-up was reduced in comparison with that observed at discharge, probably because postoperative anemia may increase its value in the early period after surgery. In the TRIBECA study Colli and colleagues (26) in 322 Trifecta implants reported at discharge mean and peak gradient values of 10.0 (8.0–15.0) and 21.0 (16.0–26.0) mmHg, and at 6–12 months of 10.0 and 20.0 mmHg, respectively.
Fiegl and colleagues (27) matched the hemodynamic performances of Trifecta (n=51) and Carpentier-Edwards Magna Ease (n=61) aortic prostheses. Trifecta valve showed lower mean pressure gradients in the early postoperative period and at 1 year. No significant differences were detected between the two prostheses with regard to left ventricular mass regression and PPM occurrence. Similarly, Minardi et al. (28) and Modi et al. (29) demonstrated the excellent hemodynamic profile of the Trifecta prosthesis for the 21 and 23 mm sizes, and a lower transvalvular gradient in comparison with Carpentier-Edwards Perimount Magna Ease valve. Hemodynamic performance of Trifecta prosthesis was also investigated in the systematic review written by Phan and coworkers (30) including 2,549 patients from 13 studies, showing a postoperative mean gradient of 9.2 mmHg, and an EOA increased up to 1.8 cm2.
In an elegant study on the fluid-dynamic results obtained comparing four pericardial aortic bioprostheses implanted in small porcine aortic roots, Tasca et al. (31) reported after Trifecta valve implantation in comparison with the other biological prostheses, i.e., Perimount Magna Ease, Mitroflow, Soprano-Armonia, better value of EOA, lower mean gradient, and lower valve resistances (P<0.001, for all comparisons). The authors draw the conclusion that biological prostheses with the pericardium outside the stent, i.e., Trifecta prosthesis, can offer a better hemodynamic performance and can be more effective in preventing PPM. In our experience, all echocardiographic parameters evaluated significantly improved during follow-up in comparison with the preoperative values (Table 2), suggesting a positive hemodynamic performance. In fact, only three patients, in which one 21 mm and two 19mm valve sizes were implanted, showed a peak trans-aortic valve gradient greater than 40 mmHg. Our echocardiographic results appeared to be satisfactory as those reported for other types of new generation prostheses (32-35).
Moreover, Rubens et al. (36) in 258 patients affected by aortic stenosis and severe left ventricular hypertrophy, showed that Trifecta aortic prosthesis was associated with a significantly increased left ventricular mass regression and improved clinical outcome at 2.5 years in comparison with Perimount Magna Ease aortic prosthesis, although all-cause mortality was similar.
In the presence of optimal performance, it is obviously expected that the freedom from structural deterioration of Trifecta aortic prosthesis should be very satisfactory. In a multicenter study performed on 710 patients undergoing Trifecta implant, Goldman et al. (37) reported a 6-year 11.0 mmHg mean gradient across all valve sizes and a 97.3% freedom from reoperation due to structural valve deterioration. Freedom from NYHA class III–IV was 95.8%. Anselmi et al. (15) on 824 consecutive Trifecta implants reported at 5 years a 98% freedom from both structural valve deterioration and reoperation. Lehmann et al. (16) reported in 918 Trifecta implants a 5-year 97.9% freedom from structural valve deterioration. These series showed satisfactory freedom from structural valve deterioration similar to that reported for the Perimount Magna Ease aortic prosthesis. At three years of follow-up we have not observed structural deterioration of the valve.
However, as also underlined by many authors (12,14,38-40), to evaluate as excellent the hemodynamic performance of the Trifecta valve, and to understand if the freedom from deterioration is equal or higher than other biological aortic valve prostheses, a long-term follow-up, i.e., at 10 years, is necessary.
PPM
In patients undergoing aortic valve replacement, especially when a biological prosthesis is implanted, PPM is not negligible and its main hemodynamic consequence is to generate high trans-aortic valve gradients through a normally functioning prosthetic valve (10). The negative impact of PPM on patient prognosis has been reported in several studies showing an increased all-cause and cardiac mortality. For these reasons, PPM minimization is becoming increasingly important when choosing which aortic valve prosthesis to implant. The incidence of moderate PPM is more frequent, ranging from 20% and 70%, whereas the incidence of severe PPM occurs more rarely, ranging from 2% and 10% (41,42). Although some studies (43,44) suggest that an increased mortality can occur only in presence of a critical level of obstruction, i.e., EOAI <0.4 cm2/m2, numerous studies showed a negative outcome also in presence of a less degree of PPM. In particular, a negative impact of the PPM on the extent of left mass ventricular regression, cardiac index, improvement of NYHA class, and cardiac event-free survival occurs in presence of an EOAI less than 0.80 or 0.75 cm2/m2 (45-47). In our study we observed that the likely favourable hemodynamic performance of the Trifecta prosthesis led to a low incidence of significant PPM. In fact, PPM was mild-to-moderate (0.82–0.85 cm2/m2) in 19% of patients, moderate (0.81–0.68 cm2/m2) in 11%, and absent in its severe degree. Lehmann et al. (16) have reported a 4.8% incidence of moderate PPM, and in no any patient was observed a severe degree of PPM. Filip and co-workers (48) in 150 aortic valve replacements found a similar incidence of moderate PPM, i.e., 27.3%. Ruggieri et al. (49) after 122 Trifecta implants reported at 3 years of follow-up 15.7% and 2.2% incidence of moderate and severe PPM, respectively. At the statistical analysis we did not found the moderate degree of PPM as a predictor of reduced survival and lack of NYHA class improvement, at least in the observed period of follow-up. Our findings are similar to those reported by Raimundo et al. (14), who reported at 5 years of follow-up 11.3% and 1.1% incidence of moderate and severe PPM, with 96% of patients in NYHA class I-II. Hernandez-Vaquero and colleagues (50) in 339 Trifecta and 963 Mitroflow implants, at 3 months reported 3.8% and 32.6% moderate PPM, and 2.1% and 9.8% severe PPM, respectively with the Trifecta and the Mitroflow valves. He concluded that Trifecta aortic prosthesis had an odds ratio of 11.9 as protector against PPM, and that the prevalence of PPM using Trifecta prosthesis appeared extraordinary low. These findings have a great importance into the clinical practice. In fact, both PARTNER and Core Valve randomized trials (51,52) demonstrated that PPM was less common after trans-catheter aortic valve replacement than after surgical aortic valve replacement, suggesting that a lower incidence of PPM after trans-catheter approach could be, at least in part, responsible for a better clinical outcome compared with conventional surgery. On the other hand, as expected, better results after TAVR could be related with the lower incidence of bleeding complications and renal dysfunction observed during TAVR in comparison with surgery.
Therefore, in contrast to other preoperative risk factors affecting outcomes of patients undergoing surgical aortic valve replacement, PPM can be prevented with the choice of the type of prosthesis correctly.
Conclusions
The present study has several limitations: it enrolled a relatively small sample size, there was the lack of a control group for the comparison of Trifecta with other biological prosthetic valves, and follow-up time is limited to a 3-year period of investigation.
In conclusion, Trifecta aortic prosthesis seems to provide a very favourable clinical outcome and hemodynamic performance. At three years, survival was negatively affected by severe coronary artery disease detected at the time of operation. During the short-term follow-up, moderate degree of PPM did not affect both survival and clinical condition. Due to low incidence of PPM and low peak and mean trans-prosthetic aortic valve gradients, third-generation Trifecta aortic prosthesis should be considered as one of the best options in the setting of the aortic valve replacement surgery. However, a long-term follow-up at least of 10 years, is mandatory to confirm the early promising data.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of the Tor Vergata University Hospital, which waived the need for patient consent. All patients gave informed surgical consent.
References
- Mariscalco G, Mariani S, Bichi S, et al. St. Jude Medical Trifecta aortic valve: results from a prospective regional multicentre registry. J Cardiothorac Surg 2015;10:169. [Crossref] [PubMed]
- Modi A, Budra M, Miskolczi S, et al. Hemodynamic performance of Trifecta: Single-center experience of 400 patients. Asian Cardiovasc Thorac Ann 2015;23:140-5. [Crossref] [PubMed]
- Permanyer E, Estigarribia AJ, Ysasi A, et al. St. Jude Medical Trifecta™ aortic valve perioperative performance in 200 patients. Interact Cardiovasc Thorac Surg 2013;17:669-72. [Crossref] [PubMed]
- Remadi JP, Levy F, Szymanski C, et al. Early hemodynamic results of aortic valve replacement with the new St. Jude Trifecta bioprosthesis. Int J Cardiol 2014;174:755-7. [Crossref] [PubMed]
- Bach DS, Patel HJ, Kolias TJ, et al. Randomized comparison of exercise haemodynamics of Freestyle, Magna Ease and Trifecta bioprostheses after aortic valve replacement for severe aortic stenosis. Eur J Cardiothorac Surg 2016;50:361-7. [Crossref] [PubMed]
- Tasca G, Vismara R, Trinca F, et al. Opening/closing pattern of Trifecta and Freestyle valves versus native aortic valve; Are stentless valves more physiologic than a stented valve? J Card Surg 2017;32:680-5. [Crossref] [PubMed]
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting morbidity and mortality after cardiac valvular interventions. J Thorac Cardiovasc Surg 2008;135:732-8. [Crossref] [PubMed]
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79-108. [Crossref] [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart 2006;92:1022-9. [Crossref] [PubMed]
- Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999;15:816-22. [Crossref] [PubMed]
- Paredes F, Estigarribia Bernal A, Permanyer E, et al. Early Experience with the Latest-Generation Biological Prosthesis, the Trifecta™ GT. J Heart Valve Dis 2017;26:721-7. [PubMed]
- Tadokoro N, Fukushima S, Shimahara Y, et al. Trifecta vs. Magna for Aortic Valve Replacement: Differences in Clinical Outcome and Valve Hemodynamics. Circ J 2018;82:2767-75. [Crossref] [PubMed]
- Raimundo R, Moreira S, Saraiva F, et al. Early and mid-term haemodynamic performance and clinical outcomes of St. Jude Medical Trifecta™ valve. J Thorac Dis 2018;10:889-98. [Crossref] [PubMed]
- Anselmi A, Ruggieri VG, Lelong B, et al. Mid-term durability of the Trifecta bioprosthesis for aortic valve replacement. J Thorac Cardiovasc Surg 2017;153:21-28.e1. [Crossref] [PubMed]
- Lehmann S, Meyer A, Schroeter T, et al. Midterm durability and hemodynamic performance of a third-generation bovine pericardial prosthetic aortic valve: The Leipzig experience. Ann Thorac Surg 2017;103:1933-9. [Crossref] [PubMed]
- Litwinowicz R, Bartus K, Drwila R, et al. In-hospital mortality in cardiac surgery patients after readmission to the intensive care unit: a single-center experience with 10,992 patients. J Cardiothorac Vasc Anesth 2015;29:570-5. [Crossref] [PubMed]
- Filip G, Litwinowicz R, Kapelak B, et al. Mid-term follow-up after suture-less aortic heart valve implantation. J Thorac Dis 2018;10:6128-36. [Crossref] [PubMed]
- Deutsch MA, Prinzing A, Fiegl K, et al. Early haemodynamic performance of a latest generation supra-annular aortic bioprosthesis: experience from a large single-centre series. Eur J Cardiothorac Surg 2016;49:1691-8. [Crossref] [PubMed]
- Levy F, Donal E, Bière L, et al. Hemodynamic performance during exercise of the new St. Jude Trifecta aortic bioprosthesis: results from a French multicenter study. J Am Soc Echocardiogr 2014;27:590-7. [Crossref] [PubMed]
- Fouquet O, Flecher E, Nzomvuama A, et al. Haemodynamic performance of the small supra-annular Trifecta bioprosthesis: results from a French multicentre study. Interact Cardiovasc Thorac Surg 2016;22:439-44. [Crossref] [PubMed]
- Ugur M, Suri RM, Daly RC, et al. Comparison of early hemodynamic performance of 3 aortic valve bioprostheses. J Thorac Cardiovasc Surg 2014;148:1940-6. [Crossref] [PubMed]
- Peterson MD, Borger MA, Feindel CM, et al. Aortic annular enlargement during aortic valve replacement: improving results over time. Ann Thorac Surg 2007;83:2044-9. [Crossref] [PubMed]
- Hanke T, Charitos EI, Paarman H, et al. Haemodynamic performance of a new pericardial aortic bioprosthesis during exercise and recovery: comparison with pulmonary autograft, stentless aortic bioprosthesis and healthy control groups. Eur J Cardiothorac Surg 2013;44:e295-e301. [Crossref] [PubMed]
- Bavaria JE, Desai ND, Cheung A, et al. The St Jude Medical Trifecta aortic pericardial valve: results from a global, multicenter, prospective clinical study. J Thorac Cardiovasc Surg 2014;147:590-7. [Crossref] [PubMed]
- Colli A, Marchetto G, Salizzoni S, et al. The TRIBECA study: (TRI)fecta (B)ioprosthesis (E)valuation versus (C)arpentier Magna-Ease in (A)ortic position. Eur J Cardiothorac Surg 2016;49:478-85. [Crossref] [PubMed]
- Fiegl K, Deutsch MA, Rondak IC, et al. Matched comparison of two different biological prostheses for complete supra-annular aortic valve replacement. Thorac Cardiovasc Surg 2015;63:459-66. [Crossref] [PubMed]
- Minardi G, Pergolini A, Zampi G, et al. St. Jude Trifecta versus Carpentier-Edwards Perimount Magna valves for the treatment of aortic stenosis: comparison of early Doppler-echocardiography and hemodynamic performance. Monaldi Arch Chest Dis 2013;80:126-32. [PubMed]
- Modi A, Pousios D, Sadeque S, et al. Early in-vivo hemodynamic comparison of supra-annular aortic bioprostheses: Trifecta versus Perimount Magna Ease. J Heart Valve Dis 2014;23:325-32. [PubMed]
- Phan K, Ha H, Phan S, et al. Early hemodynamic performance of the third generation St Jude Trifecta aortic prosthesis: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2015;149:1567-75.e1. [Crossref] [PubMed]
- Tasca G, Mhagna Z, Perotti S, et al. Impact of prosthesis-patient mismatch on cardiac events and midterm mortality after aortic valve replacement in patients with pure aortic stenosis. Circulation 2006;113:570-6. [Crossref] [PubMed]
- Sadowski J, Kapelak B, Fitzner R, et al. Sutureless aortic valve bioprosthesis '3F/ATS Enable'--4.5 years of a single-centre experience. Kardiol Pol 2009;67:956-63. [PubMed]
- Breitenbach I, Wimmer-Greinecker G, Bockeria LA, et al. Sutureless aortic valve replacement with the Trilogy Aortic valve System: multicenter experience. J Thorac Cardiovasc Surg 2010;140:878-84. [Crossref] [PubMed]
- Englberger L, Carrel TP, Doss M, et al. Clinical performance of a sutureless aortic bioprosthesis: five-year results of the 3f Enable long-term follow-up study. J Thorac Cardiovasc Surg 2014;148:1681-7. [Crossref] [PubMed]
- Bartuś K, Litwinowicz R, Kusmierczyk M, et al. Primary safety and effectiveness feasibility study after surgical aortic valve replacement with a new generation bioprosthesis: one-year outcomes. Kardiol Pol 2018;76:618-24. [PubMed]
- Rubens FD, Gee YY, Ngu BAJ, et al. Effect of aortic pericardial valve choice on outcomes and left ventricular mass regression in patients with left ventricular hypertrophy. J Thorac Cardiovasc Surg 2016;152:1291-1298.e2. [Crossref] [PubMed]
- Goldman S, Cheung A, Bavaria JE, et al. Midterm, multicenter clinical and hemodynamic results for the Trifecta aortic pericardial valve. J Thorac Cardiovasc Surg 2017;153:561-569.e2. [Crossref] [PubMed]
- Anselmi A, Ruggieri VG, Soulami RB, et al. Hemodynamic Results and Mid-term Follow-up of 850 19 to 23 mm Perimount Magna Ease Valves. Thorac Cardiovasc Surg 2018. [Epub ahead of print]. [PubMed]
- Yanagawa B, Tam DY, Hong K, et al. Magna Ease Versus Trifecta Early Hemodynamics: A Systematic Review and Meta-analysis. Innovations (Phila) 2018;13:267-72. [Crossref] [PubMed]
- Cerqueira RJ, Raimundo R, Moreira S, et al. Freedom Solo® versus Trifecta® bioprostheses: clinical and haemodynamic evaluation after propensity score matching. Eur J Cardiothorac Surg 2018;53:1264-71. [Crossref] [PubMed]
- Rao V, Jamieson WR, Ivanov J, et al. Prosthesis-patient mismatch affects survival following aortic valve replacement. Circulation 2000;102:III5-9. [Crossref] [PubMed]
- Mohty D, Girad SE, Malouf JF, et al. The impact of severe prosthesis-patient mismatch on long-term survival in patients with small St. Jude mechanical prostheses in the aortic position. J Am Coll Cardiol 2002;39:435B.
- Daneshvar SA, Rahimtoola SH. Valve prosthesis-patient mismatch. J Am Coll Cardiol 2012;60:1123-35. [Crossref] [PubMed]
- Moon MR, Lawton JS, Moazami N, et al. POINT: prosthesis-patient mismatch does not affect survival for patients greater than 70 years of age undergoing bioprosthetic aortic valve replacement. J Thorac Cardiovasc Surg 2009;137:278-83. [Crossref] [PubMed]
- Pibarot P, Dumesnil JG, Lemieux M, et al. Impact of prosthesis-patient mismatch on hemodynamic and symptomatic status, morbidity, and mortality after aortic valve replacement with a bioprosthetic heart valve. J Heart Valve Dis 1998;7:211-8. [PubMed]
- Ruel M, Rubens FD, Masters RG, et al. Late incidence and predictors of persistent or recurrent heart failure in patients with aortic prosthetic valves. J Thorac Cardiovasc Surg 2004;127:149-59. [Crossref] [PubMed]
- Nardi P, Russo M, Saitto G, et al. The Prognostic Significance of Patient-Prosthesis Mismatch after Aortic Valve Replacement. Korean J Thorac Cardiovasc Surg 2018;51:161-6. [Crossref] [PubMed]
- Filip G, Litwinowicz R, Kapelak B, et al. Patient-prosthesis mismatch after minimally invasive aortic valve replacement. Kardiol Pol 2018;76:908-10. [Crossref] [PubMed]
- Ruggieri VG, Anselmi A, Chabanne C, et al. Three-year haemodynamic performance of the St Jude Trifecta bioprosthesis. Eur J Cardiothorac Surg 2016;49:972-7. [Crossref] [PubMed]
- Hernandez-Vaquero D, Diaz R, Pascual I, et al. The Prevalence of Patient-Prosthesis Mismatch Can Be Reduced Using the Trifecta Aortic Prosthesis. Ann Thorac Surg 2018;105:144-51. [Crossref] [PubMed]
- Zorn GL, Little SH, Tadros P, et al. Prosthesis-patient mismatch in high-risk patients with severe aortic stenosis: A randomized trial of a self-expanding prosthesis. J Thorac Cardiovasc Surg 2016;151:1014-22. [Crossref] [PubMed]
- Pibarot P, Weissman NJ, Stewart WJ, et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort--a analysis. J Am Coll Cardiol. 2014;64:1323-34. [Crossref] [PubMed]