A micro-costing evaluation of lobectomy by thoracotomy versus thoracoscopy
Introduction
Lung cancer is one of the most common cancers in France with an incidence rate of slightly less than 40,000 cases per year in 2012. It is the leading cause of death for French men and the second leading cause of death from cancer for French women (1). Lobectomy is the reference procedure for the treatment of lung cancer (2). There are three different surgical procedures for lobectomy: robot assisted thoracic surgery thoracotomy and video-assisted thoracoscopy surgery (VATS). Thoracotomy is considered the benchmark treatment, but is associated with several breathing complications. Indeed, according to data from the French thoracic surgery database Epithor (http://www.epithor.net/), thoracotomy was associated with 15% of major respiratory complications in 19,178 lobectomies for lung cancer (pneumonia, atelectasis, acute respiratory distress syndrome, ventilation). These complications are responsible for a large proportion of postoperative deaths. Thereby, a minimally-invasive surgical technique called VATS has been developed over the last few years and is now considered a good alternative, in particular because it reduces the risk of complications after surgery by 10% (3).
These two techniques require different resources in terms of equipment, consumables and time during the hospitalization. Furthermore, the purchase of VATS equipment is a major investment for a hospital. For these reasons, it was interesting to conduct a micro-costing study to estimate the real costs for the two surgery techniques.
Taking into consideration the variety of the costs incurred in this particular health context, micro-costing seems to be the best way to estimate the cost of hospitalization especially since this method is considered the gold standard for economic evaluations (4). Micro-costing is thought to better estimate the actual costs of medical care and is based on the collection of cost data for each resource consumed for the patient (5). These resources are divided between consumables (including drugs), procedures and investigations, labor, medical equipment, overheads, etc.
The aim of the present study was therefore to use micro-costing methods to give an accurate estimation of the cost of a standard hospital stay for patients with lung cancer undergoing VATS or thoracotomy.
Methods
This is the first micro-costing study in France for VATS versus thoracotomy. The methodology was developed from micro-costing methodologies used in other clinical contexts as follows: first, we conducted a systematic critical review of the literature using the PubMed Medline database. The following medical subject headings were used: “micro-costing”, “microcosting” “hospitalization”, and “surgery”. Inclusion criteria for this review were as follows:
- Articles published between 2006 and 2016 in French and English;
- Studies dealing with cost analysis by micro-costing about hospitalization and/or surgery.
Articles were not selected if they met one or more of the following criteria:
- Articles without access;
- Studies conducted in several countries with various healthcare systems;
- Studies about different diseases or about one disease but more than two medical procedures;
- Articles in which data about (i) identification, (ii) quantification or (iii) valorization of cost items was not presented.
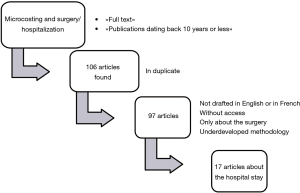
The Figure 1 presents the flow chart for the literature review.
A total of 17 articles (6-22) were selected. Most of the articles were convergent about the number of centers (single center), the number of patients (between 100 and 250), data acquired from medical records, and the methodology to collect the consumables and drugs, equipment, procedures and investigations. There was some divergence about the methodology for labor valorization. Indeed, labors costs were quantified by interview, average ward personnel per number of patients or direct observation. A score called “PflegePersonalRegelung (PPR)” (12) was used to estimate the time spent by a nurse for one patient depending on the medical characteristics of this patient. The methodology for overheads was in most cases not developed enough to be evaluated. Usually, a ratio is applied (according to the area of the sickroom, for example) because of the complexity of collecting and allocating overheads between patients precisely.
This critical analysis of the study methods enabled us to develop the following methodology to evaluate lobectomy hospitalization costs by micro-costing.
We then implemented a multicenter cost-study using a micro-costing methodology.
Fifty patients recruited from July 2015 to July 2016 in four university hospitals in France (Clermont-Ferrand, Dijon, Rouen and Paris) in a randomized controlled trial comparing lobectomy performed by VATS with lobectomy using thoracotomy for the treatment of lung cancer were included in this study. More details concerning primary study are available (23). To sum up, we included patients who underwent lobectomy for clinically proven or suspected lung cancer. Patients with a conversion during the surgery were excluded. To limit bias linked with experience of the involved surgeons in performing VATS lobectomy, a minimum of 50 should be already performed by center to pretend to be included in this protocol. We adopted the hospitals’ perspective to describe the costs associated with hospitalization for lobectomy so that we could measure the real costs of the hospitalization for lobectomy by thoracotomy or VATS. Only hospitalization costs were considered.
Data were collected prospectively using direct observations of operating rooms, and retrospectively by reviewing patients’ charts for length of stay.
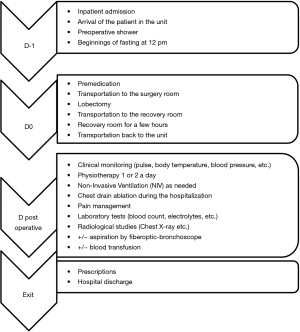
The patient’s stay at hospital was traced stage by stage from the patient’s admission to discharge, as shown in Figure 2.
The different costs were classified according the model of Drummond et al. (4). For each patient, direct observations (24-28) were recorded by a nurse. This document was used to estimate the cost of disposables, procedures and investigations (blood transfusion, pathologist), labor (surgeons, scrub technicians, circulating nurses, anesthesiologists, anesthesiologist nurses and nurses), and other materials used during the surgery period. Furthermore, the medical records for each patient included in this study were analyzed (12,13,17,18,21) to collect data for drugs, procedures and investigations, laundry and nutrition. Additionally, nurses were interviewed (11,22) to estimate the use of disposables, and labor duration. Finally, overheads were taken into account by asking for estimations from hospital managers.
Top-down micro-costing was applied for the reanimation or intensive care unit, the caregivers’ labor, the laundry and nutrition, and the overheads. Bottom-up micro-costing was used for the rest of the components such as consumables, procedures and investigations, medical and non-medical staff other than caregivers, and medical equipment (4,29).
Costs of consumables
In this section, consumables were divided into three categories: disposables used in the operating theatre, disposables used in the ward, and drugs.
The disposables used during the operation were collected prospectively by direct observation (26,27).
The number of each nursing procedure done during the hospitalization was collected to quantify disposables linked with ward activity (12,14,16,22). Drugs per unit consumed were also taken into account (12-14,16,22).
The value of consumables and the drugs were calculated by hospital’s Accounts Department and Pharmacy, respectively.
Procedures and investigations
Procedures and investigations included laboratory tests, imaging examinations, fibro-aspiration and blood transfusion. We therefore chose to focus on these. Data were collected per patient (10,12-14,18,19,22).
Data sources for unit costs about procedures and investigations were the French national cost databases called “Classification Commune des Actes Médicaux” (CCAM, Common Classification of Medical Acts) and “Nomenclature des Actes de Biologie Médicale” (NABM, List of reimbursable Test Procedures).
Personnel costs
The time spent by each category of personnel for taking care of a patient was studied.
For the operative period, the durations of the operating times were reported by a nurse. The period from the patient entering the operating room until he left for the recovery room was used to quantify labor for scrub technicians, circulating nurses, anesthesiologists, anesthesiologist nurses, and nurses (26-28). A particularity must be reported for the activity of anesthesiologists, who cover several operating rooms simultaneously and supervise many beds in the recovery room. The operative time for anesthesiologists was divided by the number of patients under their responsibility. The period from the first incision until wound closure was used to quantify time for the surgeon.
For the hospital stay, a sample of nurses from the different centers answered a questionnaire to estimate the time spent for each act. Subsequently, the number of each nursing procedure performed according to the length of stay was collected (20). Physicians’, surgeons’ and caregivers’ working hours were assigned per number of patients in the department under their responsibility. Secretarial time was estimated for the administrative registration of patients and the writing of the hospital report.
Physiotherapists’ and dieticians’ time was quantified by the number of consultations. Finally, hospital porters’ work was quantified by the number of patients transferred to the operating theatre or to another ward, such as intensive care units.
For most of the professionals, the time spent was costed according to the national median gross salary with employer’s contributions. Finally, consultations with physiotherapists and dieticians and transportation done by hospital porters were costed according to rates for these different professions.
Medical equipment
The cost of the VATS equipment and the five types of equipment (video-thoracoscopic equipment, blood pressure and vital signs monitor, morphine pump, ultrasound device and electrocardiogram) used during the hospital stay were considered (16,22). However, their use was also linked to post-operative complications.
The cost of the equipment was estimated according to the purchase price, the length of life, the number of uses (per day, or per act), and the maintenance costs.
Laundry and nutrition
An estimated 0.550 kg of laundry and two meals per bed day were used for costing purposes.
The laundry was quantified per kilogram/day, and the number of meals delivered was applied for nutrition.
The rates per kilogram or per meal were implemented respectively for laundry and meals.
Overheads
Overheads were estimated at 25% by the hospital cost accounting system. They included all the costs directly traceable to a patient (administration, energy and water, research department, buildings).
Statistical analysis
To ensure that the two groups were comparable, a descriptive analysis based on age, sex, WHO performance status, body mass index, and comorbidity was used. A Student t test was used for quantitative or binary variables and an χ2 test for qualitative variables.
The mean cost of hospitalization for lobectomy with VATS and the mean cost of hospitalization for lobectomy by thoracotomy were calculated. The two mean costs were compared using a Student test.
Because the study period was only one year, no discount rate was required (30).
The robustness of the cost results obtained was assessed through scenario and univariate sensitivity analyses. A univariate sensitivity analysis was performed for the VATS equipment.
According to French health economics recommendations, only the cost items with the greatest variation should be included in the sensitivity analysis (30). Thus, a scenario sensitivity analyses was done on parameters related to recovery room staff and overheads. Lower and upper limits for overheads are estimated by the rate of 15% used by the French ATIH “Agence Technique de l’Information sur l’Hospitalisation” (31) and the rate of 30% respectively. For the recovery room staff, limits were derived from lower and upper observations in a sample population.
Results
A total of 50 lobectomy patients were included in this study. Twenty-six patients underwent lobectomy by VATS and 24 patients by thoracotomy.
As shown in Table 1, which describes the patients’ characteristics of both groups, the comparability analysis revealed no significant difference between these two groups.

Full table
The average hospitalization duration was 7.42 days (VATS: 7.37 days vs. thoracotomy: 7.48 days). Mean operation time was 4 h 1 min (VATS: 4 h 28 min vs. thoracotomy: 3 h 34 min).
The surgery corresponded to 58.69% of the total costs for VATS and 44.58% for thoracotomy.
The costs of all lengths of stay components are shown in Table 2.
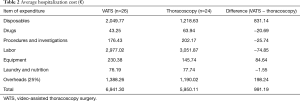
Full table
Disposables and labor were the major cost components during the hospitalization. Next, in descending order, were procedures and investigations, laundry and nutrition, equipment and drugs.
There was no major cost difference for drugs ∆ (Drugs) thoracotomy − VATS = €20.69, procedures and investigations ∆ (Procedures and investigations) thoracotomy − VATS = €25.74, and the laundry and nutrition costs ∆ (Laundry and Nutrition) thoracotomy − VATS = €1.55.
The difference in labor costs was attenuated by the fact that VATS operating theatre costs were higher and VATS ward costs were lower. As results, a difference of €74.85 favorable to VATS was obtained.
Equipment cost difference ∆ (Equipment) VATS − thoracotomy = € + 84.64 was mainly due to the video-thoracoscopic equipment component.
Similarly, cost difference for disposables ∆ (Disposables) VATS − thoracotomy = € + 831.14 was explained by the consumption of expensive consumables linked with VATS. Indeed, during VATS surgery consumables for both VATS and thoracotomy are consumed because of the risk of VATS conversion into thoracotomy.
Total costs show that hospitalization for the thoracotomy strategy was cheaper (Figure 3). All of the costs are supposed to follow a normal distribution. Despite a major cost difference between the two strategies, the t test did not show a statistically significant difference (P=0.1074). This can be explained by a large dispersion in both strategies (σVATS =2,270.99, σthoracotomy =1,974.17)
A study with more patients may show a statistically significant difference between VATS and thoracotomy costs.
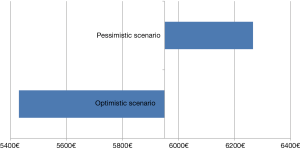
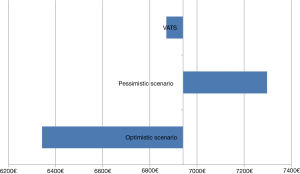
The sensitivity analysis was used to create an optimistic and pessimistic scenario represented by Tornado diagrams (Figures 4,5). Even though VATS optimistic and thoracotomy pessimistic scenarios were selected, the two scenarios did not overlap.
Discussion
No significant differences (€6,941.30 vs. €5,950.12; P>0.05) was observed between VATS and thoracotomy for lung cancer. The mean difference seems to be only attributable to operating room costs, but the data need to be confirmed in a larger sample. Moreover, differences represented by the two Tornado diagrams are mainly due to differences in overheads.
The present study is the first of its kind to compare lobectomy costs in France. Only a few foreign publications have investigated the costs of lobectomy by VATS or thoracotomy, but there was no consensus about which was the more expensive technique (32-34). The trial results reported by Alpay et al. (33) in Turkey ($3,970 vs. $3,083) cannot be compared with our results because of the discrepancies between the French and the Turkish health systems. The studies of Deen et al. (32) ($13,829.09 vs. $15,036) and Farjah et al. (34) ($35,307 vs. $37,673) both took place in the USA and conflicted with our results for the more expensive technique. However, there were major differences between their respective cost estimations. Moreover, the results from these two studies would be difficult to transpose to France because of various differences in methodologies and contexts.
The present study has several limitations. Only the hospital stay was considered, and follow-up was not taken into account. It is therefore likely that our results underestimate support for patients with lung cancer. However, this component is negligible compared with hospitalization costs, and in accordance with the recommendations, we considered that there was no difference between VATS and thoracotomy as regards the follow-up.
The retrospective nature of some of the data constitutes another limitation of the present study. Indeed, for data based on medical records, differences might have occurred between the actual resources consumed and the data collected. Information not reported in medical records may have led to an underestimation of the total cost. Unfortunately, there was no way to assess to what extent this factor may have influenced our results.
The small number of participants is one of the major limits of this study. This number is due to a low inclusion rate and that a micro-costing study implies a collection of data of high accuracy, which means a rigorous and long work that cannot be done on a large number of patients.
Average national data were used to cost investigations and procedures. We chose to break down the cost components because of feasibility concerns. Sensitivity analyses have shown the impact of this approximation.
A break-down of all overhead costs is more precise than a rate. However, the analysis was based on the assumption that the overheads did not vary across the two groups. Moreover, breaking down overhead costs is very time-consuming, which is why a rate was chosen for the analysis. However, the choice of the rate seemed to have a major impact on the cost of the hospitalization.
Concerning the quantification of nurses’ time, differences between breaking down time according to acts and average nurses’ time could be explained by the fact that talking with the patient and his/her family and friends was not taken into account in the first method. In addition, the declarative collection of data with the help of questionnaires potentially under- or overestimated the time spent by nurses on different tasks.
Experience of the involved surgeons in performing VATS was not recorded as surgeons must have performed more than 50 VATS lobectomies to participate at the study. Thus, we consider that surgeons experience has no impact on the procedure and this parameter was not integrated into statistical analysis because of the small number of participants.
More details concerning re-admission data costs and comorbidities will be added into the primary study.
Finally, some improvements need to be considered to improve the reliability of the results. Prospective data recorded for all hospitalizations can be considered, if questionnaires concerning patients are planned and collected regularly.
Nonetheless, this study had particularly strong areas. First of all, it was based on a randomized trial, thus allowing comparison between the two groups without a potential selection bias.
Then, the prospective data collected during the surgery ensured a representative estimation of the major cost components of the hospitalization.
The methodology used was based on a state-of-the-art review of the literature. We can thus expect comparable and relevant results. Moreover, this study evaluated the real costs of lobectomy by VATS and thoracotomy for lung cancer in French context. The results cannot be generalized to others contexts of care but it is an informative study for other countries as it enables to provide information about resources consumed in the strategies. Indeed, we propose a standardized processes to evaluate the cost of hospital care.
The good compliance of the medical team was a major asset in this study in that they made it possible to retrace the patient’s itinerary during the hospitalization, answered questionnaires about the time spent with each patient, and made it possible to estimate the consumables linked with nurses’ activities. Their active participation allowed us to take into account all of the items of expenditure from the hospital perspective: consumables, drugs, procedures and investigations, labor of the main members of the medical and non-medical profession, medical equipment and overheads. Moreover, the low level of missing data allowed a good estimation of the duration of nursing procedures.
The methodology of this study is a first step towards the standardization of micro-costing methodology to evaluate short stays in hospital. This is in line with the publication by the French National Authority for Health on micro-costing studies and ambulatory surgery (5). The methodology used is both feasible and shows a fairly good representation of the real costs.
This study was conducted in conjunction with a French cost-utility study called “Lungsco01”, to complete information relative to hospitalization costs. This study will make it possible to create a procedure code in the DRG to ensure its reimbursement. Indeed, CCAM rates for the lobectomy procedure range from €743.03 to €1,338.59, which are considerably lower than the surgery costs observed in this study (VATS: €3,876.49 vs. thoracotomy: € 2,466.58). Furthermore, future micro-costing studies should consider the inclusion of follow-up costs (22). Respiratory complications, which are more frequent after thoracotomy, may appear after discharge from hospital, and over time, may reduce differences between VATS and thoracotomy in terms of management costs.
According to this micro-costing study, thoracotomy seems to be the less expensive technique for the hospital. However, this finding needs to be confirmed in studies with more patients. Other considerations, like quality of life, will be taken into account with a cost-utility study. This ongoing French randomized controlled multicenter trial with the inclusion of 600 patients could validate our results and prove the efficiency of VATS before new recommendations can be drawn up. A new strategy of lobectomy by robotic is actually developing in France but we choose not to consider it in this study as this technique is used only in few hospitals. Robotic lobectomy costs could be analyzed in a future study to complete our data on lobectomy cost estimation.
Acknowledgements
The authors want to thanks the CHU of Rouen, the Cochin hospital and the Jean Perrin Center in Clermont-Ferrand for their active participation and implication in this study. This study was supported by Institut National du Cancer (INCa, French National Center Institute) and the Ministère des affaires sociales et de la santé (French Ministry of Social Affairs and Health).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Binder-Foucard F, Belot A, Delafosse P, et al. editors. Estimation nationale de l’incidence et de la mortalité par cancer en France entre 1980 et 2012. Partie 1–Tumeurs solides. Saint-Maurice: Institut de veille sanitaire, 2013. 122 p.
- Cancer primitif non à petites cellules du poumon. Le Webzine de la HAS. Accessed March 3, 2016. Available online: https://www.has-sante.fr/portail/jcms/c_1168484/
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non–small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the Economic Evaluation of Health Care Programmes. Fourth Edition. New York: Oxford University Press, 2015.
- Haute Autorité de Santé. Construction d’un outil de micro-costing en chirurgie ambulatoire. Accessed February 2, 2016. Available online: - http://www.has
- Almekhlafi MA, Hill MD, Wiebe S, et al. When is carotid angioplasty and stenting the cost-effective alternative for revascularization of symptomatic carotid stenosis? A Canadian Health System perspective. AJNR Am J Neuroradiol 2014;35:327-32. [Crossref] [PubMed]
- Bagai A, Cantor WJ, Tan M, et al. Clinical outcomes and cost implications of routine early PCI after fibrinolysis: one-year follow-up of the Trial of Routine Angioplasty and Stenting after Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction (TRANSFER-AMI) study. Am Heart J 2013;165:630-7.e2. [Crossref] [PubMed]
- Chen LA, Kim J, Boucher K, et al. Toxicity and cost-effectiveness analysis of intensity modulated radiation therapy versus 3-dimensional conformal radiation therapy for postoperative treatment of gynecologic cancers. Gynecol Oncol 2015;136:521-8. [Crossref] [PubMed]
- Dionigi G, Bacuzzi A, Boni L, et al. Visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy: what about the costs? World J Surg 2012;36:748-54. [Crossref] [PubMed]
- Fattore G, Torbica A, Susi A, et al. The social and economic burden of stroke survivors in Italy: a prospective, incidence-based, multi-centre cost of illness study. BMC Neurol 2012;12:137. [Crossref] [PubMed]
- Goossens LM, Utens CM, Smeenk FW, et al. Cost-effectiveness of early assisted discharge for COPD exacerbations in the Netherlands. Value Health 2013;16:517-28. [Crossref] [PubMed]
- Haas L, Stargardt T, Schreyoegg J, et al. Introduction of DRG-based reimbursement in inpatient psychosomatics—an examination of cost homogeneity and cost predictors in the treatment of patients with eating disorders. J Psychosom Res 2012;73:383-90. [Crossref] [PubMed]
- Haas L, Stargardt T, Schreyoegg J, et al. Inpatient costs and predictors of costs in the psychosomatic treatment of anorexia nervosa. Int J Eat Disord 2012;45:214-21. [Crossref] [PubMed]
- Haas L, Stargardt T, Schreyoegg J, et al. The trade-off between costs and quality of care in the treatment of psychosomatic patients with somatoform pain disorder. Appl Health Econ Health Policy 2013;11:359-68. [Crossref] [PubMed]
- Lee RC, Zou D, Demetrick D, et al. Costs Associated with Diffuse Large B‐Cell Lymphoma Patient Treatment in a Canadian Integrated Cancer Care Center. Value Health 2008;11:221-30. [Crossref] [PubMed]
- Nguyen TPL, Nguyen TB, Nguyen TT, et al. Direct costs of hypertensive patients admitted to hospital in Vietnam–a bottom-up micro-costing analysis. BMC Health Serv Res 2014;14:514. [Crossref] [PubMed]
- O'Brien C, Fogarty E, Walsh C, et al. The cost of the inpatient management of febrile neutropenia in cancer patients–a micro‐costing study in the Irish healthcare setting. Eur J Cancer Care (Engl) 2015;24:125-32. [Crossref] [PubMed]
- Ogata JF, Fonseca MC, Miyoshi MH, et al. Costs of hospitalization in preterm infants: impact of antenatal steroid therapy. J Pediatr (Rio J) 2016;92:24-31. [Crossref] [PubMed]
- Parkinson F, Kent SJ, Aldous C, et al. The hospital cost of road traffic accidents at a South African regional trauma centre: a micro-costing study. Injury 2014;45:342-5. [Crossref] [PubMed]
- Petis SM, Howard JL, Lanting BA, et al. In-Hospital Cost Analysis of Total Hip Arthroplasty: Does Surgical Approach Matter? J Arthroplasty 2016;31:53-8. [Crossref] [PubMed]
- Salih MR, Bahari MB, Shafie AA, et al. Medical care costs of newly diagnosed children with structural-metabolic epilepsy: A one year prevalence- based approached. Seizure 2012;21:764-9. [Crossref] [PubMed]
- Tan SS, van Putten E, Nijdam WM, et al. A microcosting study of microsurgery, LINAC radiosurgery, and gamma knife radiosurgery in meningioma patients. J Neurooncol 2011;101:237-45. [Crossref] [PubMed]
- Pagès PB, Abou Hanna H, Bertaux AC, et al. Medicoeconomic analysis of lobectomy using thoracoscopy versus thoracotomy for lung cancer: a study protocol for a multicentre randomised controlled trial (Lungsco01). BMJ Open 2017;7:e012963. [Crossref] [PubMed]
- Darlington M, Gueret P, Laissy JP, et al. Cost-effectiveness of computed tomography coronary angiography versus conventional invasive coronary angiography. Eur J Health Econ 2015;16:647-55. [Crossref] [PubMed]
- Frick KD. Microcosting quantity data collection methods. Med Care 2009;47:S76-81. [Crossref] [PubMed]
- Henry SG, Ness RM, Stiles RA, et al. A cost analysis of colonoscopy using microcosting and time-and-motion techniques. J Gen Intern Med 2007;22:1415-21. [Crossref] [PubMed]
- Ismail I, Wolff S, Gronfier A, et al. A cost evaluation methodology for surgical technologies. Surg Endosc 2015;29:2423-32. [Crossref] [PubMed]
- Sharara N, Adam V, Crott R, et al. The costs of colonoscopy in a Canadian hospital using a microcosting approach. Can J Gastroenterol 2008;22:565-70. [Crossref] [PubMed]
- Wordsworth S, Ludbrook A, Caskey F, et al. Collecting unit cost data in multicentre studies. Creating comparable methods. Eur J Health Econ 2005;6:38-44. [Crossref] [PubMed]
- Haute Autorité de Santé. Choix méthodologiques pour l’évaluation économique à la HAS. Accessed February 3, 2016. Available online: - http://www.has
- Agence Technique de l’Information sur l’Hospitalisation (ATIH). Les coûts des prises en charge à l’hôpital en médecine, chirurgie et obstétrique 2012. Accessed April 4, 2016. Available online: http://www.atih.sante.fr/sites/default/files/public/content/2563/rapport_couts_de_prise_en_charge_2012.pdf
- Deen SA, Wilson JL, Wilshire CL, et al. Defining the cost of care for lobectomy and segmentectomy: a comparison of open, video-assisted thoracoscopic, and robotic approaches. Ann Thorac Surg 2014;97:1000-7. [Crossref] [PubMed]
- Alpay L, Lacin T, Teker D, et al. A comparative cost analysis study of lobectomy performed via video-assisted thoracicsurgery versus thoracotomy in Turkey. Wideochir Inne Tech Maloinwazyjne 2014;9:409-14. [Crossref] [PubMed]
- Farjah F, Backhus LM, Varghese TK, et al. Ninety-day costs of video-assisted thoracic surgery versus open lobectomy for lung cancer. Ann Thorac Surg 2014;98:191-6. [Crossref] [PubMed]