Detection, classification, and management of rejection after lung transplantation
Introduction
Despite advances in surgical techniques and recipient and donor selection, survival following lung transplantation remains worse compared with other solid organ transplantation with a median survival of 6 years (1). Graft failure is responsible for 22.7% of deaths between 30 days and 1 year following transplant. After the first year, chronic lung allograft dysfunction (CLAD) is the leading cause of death. Multiple factors likely contribute to high rates of rejection following lung transplantation, including increased susceptibility of the lung to injury and infection as well as constant environmental exposure (2). This article will review the clinical and pathologic features of and treatment options for acute cellular rejection (ACR), acute airway rejection, antibody-mediated rejection (AMR), and CLAD.
Acute rejection
The incidence of acute rejection varies depending on the lung transplant population and data source. The registry of the International Society of Heart and Lung Transplantation (ISHLT) reports 28% of lung transplant recipients experience at least one episode of treated acute rejection in the first year following transplantation (1). The Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients report from 2016 notes a lower incidence of acute rejection at 17.1% in the first year post transplant (3). Randomized controlled studies of different immunosuppressive regimens following lung transplantation describe higher rates of rejection. In a study of mycophenylate versus everolimus in combination with cyclosporine, rates of acute rejection were 46% and 38% respectively in the first year after transplantation (4). In another study of tacrolimus and cyclosporine, 44% of all patients had at least one episode of A1 rejection and 49% had at least one episode of A2 rejection (5). Snell et al. found that everolimus significantly reduced the incidence of treated A1 rejection in the first year compared with azathioprine (7.9% vs. 32.1% respectively) (6). In a more recent study of the incidence of donor specific antibodies following lung transplantation, 64% of patients had at least 1 episode of acute rejection grade A1 or higher and 40% had one episode of rejection grade A2 or higher (7). The differences in the incidence of acute rejection in these studies are likely due to differences in protocols and timings of transbronchial biopsies, patient populations, and criteria for treatment.
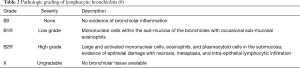
The diagnosis of acute rejection is made based on the presence of perivascular and interstitial mononuclear cell infiltrates in lung tissue (8). The diagnosis is most often made based on transbronchial biopsies obtained bronchoscopically. At least five pieces of alveolated lung parenchyma are recommended for the assessment of acute rejection (8). The histologic grade of acute cellular rejection is dependent on the intensity of the perivascular mononuclear cell cuffs and the depth of mononuclear invasion into the interstitial and alveolar spaces with grades ranging from A0 (no rejection) to A4 (severe acute rejection) (8). Table 1 summarizes the grading criteria for acute cellular rejection.
Lung transplant recipients with acute rejection may be asymptomatic or may present with non-specific symptoms such as dyspnea, cough, sputum production, and low-grade fever. Symptoms may be more frequent in patients with grade A2 or higher rejection compared with those with grade A0 or A1 (9). Although lymphocytic pleural effusions may be associated with acute rejection (10), it is also reported in patients without acute rejection (11). Multiple studies have demonstrated that acute rejection is a risk factor for the development of bronchiolitis obliterans syndrome (BOS), the major form of CLAD. Increased severity of rejection and number of episodes of rejection increase the risk of BOS (12). Multiple episodes of A1 rejection as well as even a single episode are also associated with increased risk of BOS (13,14).
Because acute rejection is a risk factor for BOS, detection and treatment are important. However, the role of surveillance bronchoscopy for screening asymptomatic patients for acute rejection remains controversial, and performance of surveillance bronchoscopies varies depending on the transplant center. A 2004 survey of lung transplant centers in North America found that 69% of centers performed surveillance bronchoscopies (15). However, the data supporting surveillance bronchoscopies is mixed. In a single-center study in which some patients were monitored by surveillance bronchoscopy and others underwent clinically-indicated bronchoscopy, Valentine et al. found no differences in acute rejection, infection, or bronchiolitis obliterans-free survival between the two groups (16). More bronchoscopies were performed in the surveillance group compared with the clinically indicated group. In another prospective study of all bronchoscopic procedures at a single center, complication rates over 12 months were similar in patients who underwent surveillance bronchoscopies and those who underwent clinically indicated procedures, and approximately 18 percent of patients undergoing surveillance bronchoscopy were found to have acute rejection grade A2 or higher (17). Surveillance bronchoscopies may also detect other clinically relevant diagnoses such as infection (16,17). Centers who do not perform routine surveillance bronchoscopies may use lower thresholds to determine the need for clinically indicated bronchoscopies.
Lymphocytic bronchiolitis is characterized by airway inflammation without identifiable cause, such as co-existing infection. As shown in Table 2, lymphocytic bronchiolitis is graded as no airway inflammation (B0), low grade small airway inflammation (B1R), and high grade small airway inflammation (B2R) (8). Because there may be inadequate sampling of small airways in transbronchial biopsies, an ungradable category (BX) also exists for biopsies limited by sampling or processing problems. Lymphocytic bronchiolitis, independent of ACR, has been found to be a significant risk factor for both the development of BOS and death (18). Treatment of isolated lymphocytic bronchiolitis is controversial.
In general, there is consensus that acute cellular rejection grades A2 or higher require treatment. Treatment is generally with pulse corticosteroids, but there are no studies to define the optimal amount and duration of therapy. Most centers use intravenous methylprednisolone of 10–15 mg/kg daily or 500 to 1,000 mg daily for 3 days. An oral prednisone taper may follow. The management of asymptomatic minimal acute rejection (grade A1) remains controversial, despite its association with the development of BOS. Follow-up bronchoscopy may be performed to follow-up acute rejection in order to assess response to therapy or if untreated, rule out progression to a higher grade. Studies of the value of follow-up biopsies have shown that 26–44% of patients with moderate ACR have persistent rejection (19,20). There is no accepted, standardized regimen for treatment of persistent or refractory acute rejection. Reported approaches include additional intravenous glucocorticoids, antithymocyte globulin, alemtuzumab, total lymphoid radiation, and extracorporeal photopheresis (ECP) (21-23).
Antibody mediated rejection
AMR is a well-recognized entity following heart and kidney transplantation. Deposition of the complement split product C4d on the capillary endothelium has been suggested as a marker of AMR in other organ transplants. However, C4d immunofluorescence staining on lung tissue is a less reliable test, because of high background from non-specific binding, frequent focal staining, and presence of C4d deposition in infection and reperfusion injury (24). In addition, there is an unclear relationship between the presence of donor specific antibodies with graft damage and dysfunction, leading to difficulty in establishing a diagnosis in AMR. In 2016, a consensus document on AMR was published by ISHLT in order to standardize the diagnosis of AMR (25). This consensus statement divides AMR into two subtypes: clinical, defined by measurable allograft dysfunction, and subclinical, characterized by normal allograft function. AMR is then further sub-categorized into definite, probable, and possible based on the number of diagnostic criteria (25), with the greater number of criteria increasing diagnostic certainty. These criteria for definite AMR include: (I) exclusion of other causes such as infection; (II) histopathologic features; (III) presence of DSA; and (IV) positive C4d staining. Histopathologic features of AMR are non-specific and include neutrophilic capillaritis, neutrophil margination, acute lung injury with or without diffuse alveolar damage, and arteritis (26). A diagnosis of probable AMR lacks one criteria, a diagnosis of possible AMR lacks two criteria.
The true incidence and outcomes of AMR are unknown, since many reports of AMR were published before the current diagnostic criteria were established. In one center’s series of 21 patients published prior to the most recent consensus statement, 21 cases of AMR were diagnosed in 501 lung transplant procedures (27). Of these 21 cases, 15 patients improved and were discharged from the hospital. Thirteen of the 14 discharged patients without pre-existing CLAD developed CLAD, and the median survival after diagnosis of AMR was 593 days. Patients who cleared DSA after therapy had better survival than those who did not. Another case series reported two patients with three markers of AMR out of 62 patients. Both of these patients had clinical improvement after treatment (28).
There is no standardized treatment for AMR, and there have been no randomized trials or head-to-head trials investigating AMR treatment. Common regimens for AMR treatment target the B-cell pathway and aim to deplete circulating antibodies and suppress B cells to prevent additional antibody formation. These regimens include intravenous immune globulin (IVIG), plasmapheresis, and rituximab alone or in combination. Additional agents such as carfilzomib or bortezomib, both of which are proteasome inhibitors, or eculizumab, an antibody targeting the C5 complement protein, may also be added (27,29).
CLAD
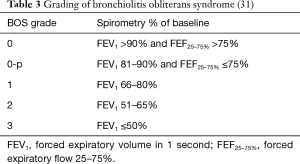
Chronic rejection is the major barrier to long-term survival following lung transplantation (1). CLAD includes both obliterative bronchiolitis (OB)/BOS and restrictive allograft dysfunction (RAS). OB is characterized by scarring or filling of the airway lumen seen on histopathology, leading to small airway obstruction. Because OB is difficult to diagnose by transbronchial biopsies or other non-invasive tests, BOS is clinically defined by pulmonary function measurements (30). It is defined by a decrease in the forced expiratory volume in 1 second (FEV1) of at least 20% from the best baseline value, where the best baseline value is the average of the two highest post-transplant values obtained at least 3 weeks apart (31). Additionally, identifiable causes such as acute rejection, infection, and anastomotic issues must be excluded. As seen in Table 3, BOS is graded based on the degree of decrease in FEV1. Approximately 50% of lung transplant recipients develop BOS within 5 years after transplant (1). Median survival after a diagnosis of BOS is 3–5 years.
Although BOS is its most common form, CLAD is a heterogeneous condition with different phenotypes. RAS is being increasingly recognized as a second phenotype of CLAD. There are no universally accepted diagnostic criteria for RAS. In the first description of RAS, Sato et al. defined RAS as irreversible decline of FEV1 to less than 80% of baseline in combination with an irreversible decline in total lung capacity (TLC) to less than 90% of baseline (32). RAS was further characterized by radiographic findings of upper lobe predominant fibrosis and histologically by diffuse alveolar damage and fibrosis in the alveolar interstitium, visceral pleural, and interlobular septa. Pleuroparenchymal fibroelastosis, with and without concomitant OB, was later identified as the major histopathologic finding in RAS (33). Verleden et al. (34) identified a group of patients with insufficient TLC data to diagnose RAS based on TLC, but found that these patients had a decrease in forced vital capacity (FVC) with a normal FEV1/FVC ratio. The same group later proposed that a decrease in TLC ≥10% or a decrease in FVC ≥20% if no TLC was available could be used to diagnose RAS (35). Together, these studies determined that RAS accounts for approximately 25% to 35% of CLAD cases and has a worse prognosis compared with BOS with a median survival of only 6–18 months after diagnosis (32,35,36). The BOS and RAS phenotypes of CLAD are not mutually exclusive, and patients may evolve from one phenotype to the other.
Multiple factors have been identified as risk factors for the development of BOS. As discussed above, acute cellular rejection and lymphocytic bronchiolitis are risk factors for BOS and have also been identified as risk factors for the development of RAS (37). Other risk factors associated with BOS include primary graft dysfunction (38,39), presence of de novo donor specific antibodies (40,41), and presence of gastroesophageal reflux disease (42). Bacterial, fungal, and viral infections and colonization are also associated with BOS, particularly with Pseudomonas aeruginosa (43,44), Aspergillus (45,46), and cytomegalovirus (47).
Azithromycin, an immunomodulatory macrolide antibiotic, has been studied in the prevention and treatment of CLAD. Thirty to 83% of patients with BOS have improvement in FEV1 when treated with azithromycin (48). A proportion of patients continue to have decline in FEV1 despite treatment (49,50). Responders tend to receive azithromycin earlier after transplantation (50). Some studies also suggest that responders have bronchoalveolar lavage neutrophilia (50,51), though other studies do not support this finding (49). In a small, randomized, controlled trial, prophylactic azithromycin led to decreased incidence of BOS and longer BOS-free survival (52). Azithromycin has also been shown to improve mortality in lung transplant recipients with BOS stage 1, but not stage 2 (53). In a post-hoc analysis with long-term follow-up of patients receiving azithromycin and placebo, use of azithromycin delayed the development of CLAD compared with placebo (54).
Montelukast, a cysteinyl leukotriene, has recently been studied as a potential treatment for CLAD. In a retrospective, single center study, treatment of lung transplant recipients with established CLAD with montelukast 10 mg daily attenuated the rate of decline of FEV1 (55). Sixty-one percent of patients were free from CLAD progression, defined by a less than 10% decrease or increase in FEV1. However, a small, randomized controlled study by the same group failed to demonstrate a survival benefit or difference in rate of change of FEV1 with montelukast compared with placebo (56). Findings in this study were likely limited by a sample size of only 30 patients.
ECP, a procedure which removes lymphocytes from peripheral blood, exposes them to a photosensitizing agent followed by UV light, then returns the treated blood to the patient, has also been used in the treatment of BOS. ECP has been shown to reduce the rate of decline of lung function and improve survival in patients with BOS (57). A randomized study of ECP in Medicare recipients with BOS is currently enrolling (NCT02181257, www.clinicaltrials.gov). Other therapies for BOS include antithymocyte globulin, total lymphoid irradiation, and alemtuzumab, an anti-CD52 antibody (58). Finally, retransplantation is an option for patients with progressive CLAD despite treatment. Retransplantation has worse survival compared with initial transplant and a higher incidence of BOS in the first 5 years following transplant (1). Furthermore, patients with RAS phenotype have worse survival after retransplantation when compared with patients with BOS (59). Therefore, careful patient selection is important when considering retransplantation for CLAD.
Conclusions
Rejection remains a significant problem following lung transplantation. Acute cellular rejection, lymphocytic bronchiolitis, and AMR are all risk factors for the subsequent development of CLAD, the leading cause of death following the first year after transplantation. More information is needed to better identify and further refine phenotypes of CLAD, especially since treatment efficacy and prognosis differ for RAS compared with BOS. Randomized controlled trials are also needed to differentiate the effect of therapy from the natural course of the disease.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chambers DC, Yusen RD, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1047-59. [Crossref] [PubMed]
- Martinu T, Chen DF, Palmer SM. Acute rejection and humoral sensitization in lung transplant recipients. Proc Am Thorac Soc 2009;6:54-65. [Crossref] [PubMed]
- Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2016 Annual Data Report: Lung. Am J Transplant 2018;18 Suppl 1:363-433. [Crossref] [PubMed]
- Glanville AR, Aboyoun C, Klepetko W, et al. Three-year results of an investigator-driven multicenter, international, randomized open-label de novo trial to prevent BOS after lung transplantation. J Heart Lung Transplant 2015;34:16-25. [Crossref] [PubMed]
- Hachem RR, Yusen RD, Chakinala MM, et al. A randomized controlled trial of tacrolimus versus cyclosporine after lung transplantation. J Heart Lung Transplant 2007;26:1012-8. [Crossref] [PubMed]
- Snell GI, Valentine VG, Vitulo P, et al. Everolimus versus azathioprine in maintenance lung transplant recipients: an international, randomized, double-blind clinical trial. Am J Transplant 2006;6:169-77. [Crossref] [PubMed]
- Hachem RR, Kamoun M, Budev MM, et al. Human leukocyte antigens antibodies after lung transplantation: Primary results of the HALT study. Am J Transplant 2018;18:2285-94. [Crossref] [PubMed]
- Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26:1229-42. [Crossref] [PubMed]
- De Vito Dabbs A, Hoffman LA, Iacono AT, et al. Are symptom reports useful for differentiating between acute rejection and pulmonary infection after lung transplantation? Heart Lung 2004;33:372-80. [Crossref] [PubMed]
- Judson MA, Handy JR, Sahn SA. Pleural effusion from acute lung rejection. Chest 1997;111:1128-30. [Crossref] [PubMed]
- Shitrit D, Izbicki G, Fink G, et al. Late postoperative pleural effusion following lung transplantation: characteristics and clinical implications. Eur J Cardiothorac Surg 2003;23:494-6. [Crossref] [PubMed]
- Husain AN, Siddiqui MT, Holmes EW, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 1999;159:829-33. [Crossref] [PubMed]
- Hachem RR, Khalifah AP, Chakinala MM, et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation 2005;80:1406-13. [Crossref] [PubMed]
- Hopkins PM, Aboyoun CL, Chhajed PN, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med 2004;170:1022-6. [Crossref] [PubMed]
- Levine SM. Transplant/Immunology Network of the American College of Chest P. A survey of clinical practice of lung transplantation in North America. Chest 2004;125:1224-38. [Crossref] [PubMed]
- Valentine VG, Gupta MR, Weill D, et al. Single-institution study evaluating the utility of surveillance bronchoscopy after lung transplantation. J Heart Lung Transplant 2009;28:14-20. [Crossref] [PubMed]
- McWilliams TJ, Williams TJ, Whitford HM, et al. Surveillance bronchoscopy in lung transplant recipients: risk versus benefit. J Heart Lung Transplant 2008;27:1203-9. [Crossref] [PubMed]
- Glanville AR, Aboyoun CL, Havryk A, et al. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med 2008;177:1033-40. [Crossref] [PubMed]
- Guilinger RA, Paradis IL, Dauber JH, et al. The importance of bronchoscopy with transbronchial biopsy and bronchoalveolar lavage in the management of lung transplant recipients. Am J Respir Crit Care Med 1995;152:2037-43. [Crossref] [PubMed]
- Aboyoun CL, Tamm M, Chhajed PN, et al. Diagnostic value of follow-up transbronchial lung biopsy after lung rejection. Am J Respir Crit Care Med 2001;164:460-3. [Crossref] [PubMed]
- Reams BD, Musselwhite LW, Zaas DW, et al. Alemtuzumab in the treatment of refractory acute rejection and bronchiolitis obliterans syndrome after human lung transplantation. Am J Transplant 2007;7:2802-8. [Crossref] [PubMed]
- Valentine VG, Robbins RC, Wehner JH, et al. Total lymphoid irradiation for refractory acute rejection in heart-lung and lung allografts. Chest 1996;109:1184-9. [Crossref] [PubMed]
- Villanueva J, Bhorade SM, Robinson JA, et al. Extracorporeal photopheresis for the treatment of lung allograft rejection. Ann Transplant 2000;5:44-7. [PubMed]
- Roden AC, Aisner DL, Allen TC, et al. Diagnosis of Acute Cellular Rejection and Antibody-Mediated Rejection on Lung Transplant Biopsies: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2017;141:437-44. [Crossref] [PubMed]
- Levine DJ, Glanville AR, Aboyoun C, et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2016;35:397-406. [Crossref] [PubMed]
- Berry G, Burke M, Andersen C, et al. Pathology of pulmonary antibody-mediated rejection: 2012 update from the Pathology Council of the ISHLT. J Heart Lung Transplant 2013;32:14-21. [Crossref] [PubMed]
- Witt CA, Gaut JP, Yusen RD, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant 2013;32:1034-40. [Crossref] [PubMed]
- Daoud AH, Betensley AD. Diagnosis and treatment of antibody mediated rejection in lung transplantation: a retrospective case series. Transpl Immunol 2013;28:1-5. [Crossref] [PubMed]
- Ensor CR, Yousem SA, Marrari M, et al. Proteasome Inhibitor Carfilzomib-Based Therapy for Antibody-Mediated Rejection of the Pulmonary Allograft: Use and Short-Term Findings. Am J Transplant 2017;17:1380-8. [Crossref] [PubMed]
- Chamberlain D, Maurer J, Chaparro C, et al. Evaluation of transbronchial lung biopsy specimens in the diagnosis of bronchiolitis obliterans after lung transplantation. J Heart Lung Transplant 1994;13:963-71. [PubMed]
- Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002;21:297-310. [Crossref] [PubMed]
- Sato M, Waddell TK, Wagnetz U, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant 2011;30:735-42. [Crossref] [PubMed]
- Ofek E, Sato M, Saito T, et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod Pathol 2013;26:350-6. [Crossref] [PubMed]
- Verleden GM, Vos R, Verleden SE, et al. Survival determinants in lung transplant patients with chronic allograft dysfunction. Transplantation 2011;92:703-8. [Crossref] [PubMed]
- Van Herck A, Verleden SE, Sacreas A, et al. Validation of a post-transplant chronic lung allograft dysfunction classification system. J Heart Lung Transplant 2019;38:166-73. [Crossref] [PubMed]
- Verleden SE, Ruttens D, Vandermeulen E, et al. Restrictive chronic lung allograft dysfunction: Where are we now? J Heart Lung Transplant 2015;34:625-30. [Crossref] [PubMed]
- Verleden SE, Ruttens D, Vandermeulen E, et al. Bronchiolitis obliterans syndrome and restrictive allograft syndrome: do risk factors differ? Transplantation 2013;95:1167-72. [Crossref] [PubMed]
- Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2007;175:507-13. [Crossref] [PubMed]
- Huang HJ, Yusen RD, Meyers BF, et al. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant 2008;8:2454-62. [Crossref] [PubMed]
- Palmer SM, Davis RD, Hadjiliadis D, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation 2002;74:799-804. [Crossref] [PubMed]
- Girnita AL, Duquesnoy R, Yousem SA, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant 2005;5:131-8. [Crossref] [PubMed]
- King BJ, Iyer H, Leidi AA, et al. Gastroesophageal reflux in bronchiolitis obliterans syndrome: a new perspective. J Heart Lung Transplant 2009;28:870-5. [Crossref] [PubMed]
- Botha P, Archer L, Anderson RL, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation 2008;85:771-4. [Crossref] [PubMed]
- Vos R, Vanaudenaerde BM, Geudens N, et al. Pseudomonal airway colonisation: risk factor for bronchiolitis obliterans syndrome after lung transplantation? Eur Respir J 2008;31:1037-45. [Crossref] [PubMed]
- Weigt SS, Elashoff RM, Huang C, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant 2009;9:1903-11. [Crossref] [PubMed]
- Weigt SS, Copeland CA, Derhovanessian A, et al. Colonization with small conidia Aspergillus species is associated with bronchiolitis obliterans syndrome: a two-center validation study. Am J Transplant 2013;13:919-27. [Crossref] [PubMed]
- Snyder LD, Finlen-Copeland CA, Turbyfill WJ, et al. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med 2010;181:1391-6. [Crossref] [PubMed]
- Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 2014;44:1479-503. [Crossref] [PubMed]
- Federica M, Nadia S, Monica M, et al. Clinical and immunological evaluation of 12-month azithromycin therapy in chronic lung allograft rejection. Clin Transplant 2011;25:E381-9. [Crossref] [PubMed]
- Vos R, Vanaudenaerde BM, Ottevaere A, et al. Long-term azithromycin therapy for bronchiolitis obliterans syndrome: divide and conquer? J Heart Lung Transplant 2010;29:1358-68. [Crossref] [PubMed]
- Gottlieb J, Szangolies J, Koehnlein T, et al. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation 2008;85:36-41. [Crossref] [PubMed]
- Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J 2011;37:164-72. [Crossref] [PubMed]
- Jain R, Hachem RR, Morrell MR, et al. Azithromycin is associated with increased survival in lung transplant recipients with bronchiolitis obliterans syndrome. J Heart Lung Transplant 2010;29:531-7. [Crossref] [PubMed]
- Ruttens D, Verleden SE, Vandermeulen E, et al. Prophylactic Azithromycin Therapy After Lung Transplantation: Post hoc Analysis of a Randomized Controlled Trial. Am J Transplant 2016;16:254-61. [Crossref] [PubMed]
- Vos R, Eynde RV, Ruttens D, et al. Montelukast in chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant 2019;38:516-27. [Crossref] [PubMed]
- Ruttens D, Verleden SE, Demeyer H, et al. Montelukast for bronchiolitis obliterans syndrome after lung transplantation: A randomized controlled trial. PLoS One 2018;13:e0193564. [Crossref] [PubMed]
- Jaksch P, Scheed A, Keplinger M, et al. A prospective interventional study on the use of extracorporeal photopheresis in patients with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant 2012;31:950-7. [Crossref] [PubMed]
- Benden C, Haughton M, Leonard S, et al. Therapy options for chronic lung allograft dysfunction-bronchiolitis obliterans syndrome following first-line immunosuppressive strategies: A systematic review. J Heart Lung Transplant 2017;36:921-33. [Crossref] [PubMed]
- Verleden SE, Todd JL, Sato M, et al. Impact of CLAD Phenotype on Survival After Lung Retransplantation: A Multicenter Study. Am J Transplant 2015;15:2223-30. [Crossref] [PubMed]