
Standard dose osimertinib for erlotinib refractory T790M-negative EGFR-mutant non-small cell lung cancer with leptomeningeal disease
Introduction
The spread of non-small cell lung cancer (NSCLC) to the central nervous system (CNS) is a frequent occurrence and is universally associated with poor outcomes (1). Approximately 10% of patients with NSCLC develop leptomeningeal disease. This harbours a poor prognosis with a median overall survival (OS) of 2.5 months from diagnosis (2). Activating mutations of the epidermal growth factor receptor (EGFR) are associated with an increased cumulative risk of developing CNS disease, with leptomeningeal disease being particularly prevalent (3).
Treatment of de novo leptomeningeal disease in EGFR-mutated NSCLC has been hampered by poor blood-brain barrier penetration from the standard daily dosing of first-generation EGFR tyrosine kinase inhibitors (TKIs), erlotinib and gefitinib (4). Several therapeutic strategies have been employed in order to overcome this, including dose escalation (5,6), high-dose pulsed weekly administration (7) and concurrent 1st generation TKI with whole brain radiotherapy (8), all with varying rates of success. More recently, 3rd generation TKIs with greater CNS penetration have been developed, with the aim of improving control and preventing metastatic disease in the brain. In the FLAURA study, the progression free survival (PFS) of osimertinib, a 3rd generation TKI was superior to erlotinib and gefitinib, both in the overall population and in the subset of patients with baseline untreated, asymptomatic CNS metastases [median PFS 15.2 (12.2–21.4) months with osimertinib vs. 9.6 (7.0–12.4) months with 1st generation TKIs, hazard ratio 0.47 (95% CI, 0.30–0.74), P<0.001] (9). The findings from this study led to the FDA approval of osimertinib in the 1st line setting of patients with metastatic EGFR-mutated NSCLC in April 2018.
However, there is a significant proportion of patients who remain on 1st generation TKIs due to limited regulatory approvals for first-line osimertinib in other countries and patients who preceded the FLAURA study. The majority of these patients experience disease progression around the 12-month mark, and approximately 30% of patients develop disease progression in the CNS (10). A proportion of these patients develop CNS-only progression, with a subset being leptomeningeal disease. In patients with systemic disease progression, the most common mechanism of resistance to 1st generation TKIs is development of the EGFR T790M mutation, but other resistance mechanisms include amplification of HER2, MET, mutations of PIK3CA and BRAF and small-cell transformation (11).
CNS disease progression on 1st generation TKIs is a unique entity. In one study of patients whose disease had progressed on a 1st generation TKI, the EGFR T790M mutation was found in the lung biopsy samples of 12 patients, but 10 of them had EGFR T790M-negative matched CNS biopsies (12). A postulated cause for this is the ‘pharmacokinetic resistance’ of 1st generation TKIs in the CNS due to an inability to penetrate the blood brain barrier and achieving adequate inhibitory levels. A number of other groups have reported their findings on cerebrospinal fluid (CSF) analysis using polymerase chain reaction (PCR) methods and also found low rates of the EGFR T790M mutation in CSF on progression of a 1st generation TKI (13,14).
The BLOOM study, an open-label, multicentre, phase I study, was recently reported (15,16). This trial had two separate study arms; one with AZD3759, a reversible EGFR TKI designed to have improved CNS penetrance for patients with brain metastases, and one arm with double-dose daily (160 mg) osimertinib in EGFR T790M-unselected and EGFR T790M-positive patients with leptomeningeal disease. In the AZD3759 cohort, the objective response rate was 52% (11/21) in patients with measurable brain metastases and in the 160 mg daily osimertinib cohort, the ORR was 33% (7/21) in patients with leptomeningeal disease. Accrual to the osimertinib arm of the study for patients with EGFR T790M-positive disease was completed in August 2018 (NCT02228369) and results are awaited.
The incidence of the EGFR T790M mutation in the CSF is low and patients with 1st generation EGFR TKI refractory disease with CNS relapse often have poor performance status. In addition, their therapeutic options, which include radiotherapy and chemo-immunotherapy, have modest CNS responses and are associated with higher toxicity rates. Given the potential of osimertinib to overcome the pharmacokinetic failure seen with 1st generation TKIs, with possible improved efficacy and toxicity than standard therapies, we sought to explore its role in the treatment of CNS relapse. In this manuscript, we describe a patient who was treated at our institution with standard-dose osimertinib in the context of erlotinib refractory EGFR T790M-negative leptomeningeal disease and discuss our clinical experience with droplet digital PCR (ddPCR) in eight patients with leptomeningeal disease, documenting clonal heterogeneity in paired plasma and CSF sampling.
Methods
This study was approved by the Austin Health Human Research Ethics Committee (No. H2012/04446). Nine patients with EGFR mutated NSCLC who had progressed on an EGFR TKI and developed MRI evidence of leptomeningeal disease from our institution between January 2016 and August 2018 were included in this study. All patients had a diagnosis of metastatic lung adenocarcinoma with a previously documented activating EGFR mutation and CSF cytology confirming adenocarcinoma. Clinical information both preceding and subsequent to ddPCR testing was retrospectively collected. OS was evaluated from the date of diagnosis of leptomeningeal disease until the date of death or censoring on the 1st August 2018.
With written informed consent, paired CSF and plasma were obtained from eligible patients and subjected to cytological examination and EGFR mutation analysis using ddPCR (17). Cell free DNA was extracted from 1 mL of CSF or 4 mL of plasma using the QIAamp Circulating Nucleic Acid Kit (Qiagen) according to the manufacturer’s guidelines. The DNA was eluted in 50–100 µL and tested for EGFR L858R, EGFR exon 19 deletions, EGFR T790M and EGFR C797S mutations using droplet digital PCR (Bio-Rad). The ddPCR master mix included the commercially available EGFR L858R assay primer/probe mix (Bio-Rad; Assay ID-dHsaMDV2010021) or EGFR T790M assay primer/probe mix (Bio-Rad; Assay ID-dHsaMDV2010019) with 0.9X of ddPCR supermix for probes (2X: No dUTP) and molecular grade water to a total volume of 13 µL. Ten µL of cell free DNA was added to make a total 23 µL reaction mix. The ddPCR master mix for the EGFR exon 19 deletion and EGFR C797S assays included in-house developed primers and probes, 0.9X of ddPCR supermix for probes [2X: No dUTP (Bio-Rad)] with molecular grade water to a total volume of 13 µL. Ten µL of cell free DNA was added to make a total 23 µL reaction mix.
For droplet generation, 20 µL of the reaction mix was added to DG8 cartridges with 70 µL of droplet generation oil for probes. Droplets were then manually transferred to Twin-tec Eppendorf 96 well plates (Eppendorf) for thermal cycling on a Bio-Rad C1000 thermal cycler. The PCR cycling conditions were; 1 min activation at 95 °C followed by 40 cycles of 30 seconds at 94 °C and 60 seconds at 55 °C. A 10 min hold at 98 °C followed by storage at 12 °C until the plate was read on the QX200 droplet reader. The ddPCR results were analysed using the QuantaSoft™ version 1.7.4 (Bio-Rad) software.
Quantitative levels of the primary EGFR, T790M and C797S mutations for each patient are represented in Figure 1. Patient 1 is presented as a case study and the remaining 8 patients presented as a case series.

Results
Case study
A 79-year-old lady of Italian heritage (Patient 1) with a history of resected Stage 1B (T2aN0) lung adenocarcinoma with an EGFR exon 19 deletion mutation and who had completed four cycles of adjuvant carboplatin-vinorelbine chemotherapy in January 2011, presented to our institution in April 2016 with 3 months of worsening headaches and nausea. She was previously well and her only relevant medical history was polymyalgia rheumatica, for which she was on 5 mg daily oral prednisolone.
She was admitted under the neurology unit for investigations and rapidly deteriorated with increasing drowsiness and subsequently obtundation. She was transferred to the intensive care unit where she underwent an MRI brain and lumbar puncture. The MRI brain demonstrated an enhancing left occipital lesion, subtle leptomeningeal enhancement and hydrocephalus (Figure 2A). The lumbar puncture confirmed metastatic lung adenocarcinoma on cytology and ddPCR of the CSF demonstrated high levels (127 copies/mL) of the EGFR exon 19 deletion mutation. A paired plasma sample was negative for the EGFR mutation and in keeping with this, her CT scans did not demonstrate extra-cranial disease.
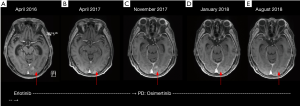
Due to the degree of obtundation and evidence of hydrocephalus, a ventriculoperitoneal shunt was inserted, leading to a partial improvement in her condition. She was ineligible for the BLOOM study due to the shunt and her performance status, and was started on 1,500 mg weekly pulsed erlotinib in April 2016. Her condition rapidly improved and she was discharged home two weeks later. She continued to tolerate and respond to pulsed weekly erlotinib for 19 months (Figure 2B) until she started developing increasing confusion, headaches and drowsiness in November 2017, once again associated with a deterioration in her functional status. An MRI brain demonstrated disease progression with leptomeningeal enhancement in the left medial occipital lobe (Figure 2C). A repeat lumbar puncture and ddPCR analysis of her CSF demonstrated a 25-fold increase in her EGFR exon 19 deletion mutation (3,197 copies/mL), compared to baseline. There was no EGFR T790M mutation identified and once again her plasma sample was negative for both the activating primary EGFR driver and T790M mutations.
Due to her rapidly deteriorating performance status and concerns regarding her ability to tolerate radiotherapy or chemo-immunotherapy, we commenced her on standard 80 mg daily osimertinib. Within 1 week, her functional status improved dramatically and within 1 month, she was able to resume her regular hydrotherapy exercises. Repeat MRI brain 6 weeks later showed improvement of the leptomeningeal enhancing changes (Figure 2D) and she continues on osimertinib to date without any complications. Her most recent MRI in August 2018 (Figure 2E) confirms ongoing disease response, 9 months after commencing osimertinib.
Case series
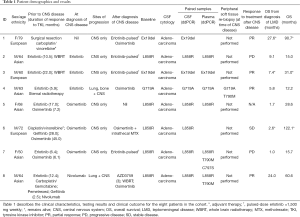
The characteristics and clinical courses of the eight patients (five males, three females) in this series are summarized in Table 1. The median age was 64 years (ranges from 50 to 79 years) and 5/8 (63%) were of Asian ethnicity. All patients had histologically confirmed lung adenocarcinoma and a primary EGFR mutation (two with exon 19 deletion, five with L858R and one with G719A). In six patients, the primary EGFR mutation identified at the time of CNS disease, was the same in CSF and plasma. In two patients, there was no identifiable primary EGFR mutation in plasma at the time of CNS disease confirmation.
Full table
Patients 2, 3 and 4 were on erlotinib when they developed leptomeningeal disease. Both patients 2 and 3 did not have detectable EGFR T790M mutation in their plasma or CSF and were switched to weekly pulsed erlotinib. Patient 2 did not have a CNS response and died 9.1 months after diagnosis of leptomeningeal disease, while patient 3 had a partial response in the CNS and remains alive 7.4 months from leptomeningeal disease diagnosis. Patient 4 also did not have detectable EGFR T790M mutation in plasma or CSF but had a T790M mutation on tissue biopsy. He was commenced on osimertinib, with a partial response both extra and intra-cranially, but this was not durable and he died from rapid disease progression 5.8 months after diagnosis of leptomeningeal disease.
Patients 5, 6 and 7 experienced progression of leptomeningeal disease while being treated with osimertinib. All three patients had received a 1st generation TKI prior to osimertinib. In patient 5, we identified both the primary EGFR mutation and T790M mutation in CSF and plasma but we could not identify the resistant C797S mutation in either. The patient rapidly deteriorated and died 1.7 months later. Patient 6 had the primary EGFR mutation in CSF but not in plasma, and the T790M mutation and C797S mutations were not detectable in either plasma or CSF. The patient was commenced on intrathecal methotrexate in combination with osimertinib and remains clinically stable 2.6 months later. Patient 7 had the primary EGFR, T790M and C797S mutations identified in plasma but only the primary EGFR mutation was detected in CSF. To determine the cis or trans allelic relationship of the EGFR C797S with T790M mutation, the patient’s plasma DNA was deep sequenced using the Oncomine Lung cfDNA Assay (Thermo Fisher Scientific). Both the EGFR C797S and T790M mutations were detected on the same DNA strand, indicating the cis-allelic relationship. She did not respond to a switch in therapy to pulsed weekly erlotinib and died 1 month later.
Patient 8 was on nivolumab when he was found to have evidence of CNS disease. He was recruited to the AZD3759 dose escalation arm of the BLOOM study and had a mixed disease response, with CNS response but disease progression in the lungs. He was found to have the primary EGFR mutation and T790M mutation in plasma but only the primary EGFR mutation in CSF. He was switched to osimertinib and responded both extra and intra-cranially for 12 months before disease progression, and died 24 months after diagnosis of leptomeningeal disease.
Discussion
In the era of precision medicine, detailed analysis of both tissue and liquid biopsies has become a component of routine clinical practice in order to determine the optimal therapeutic option for patients. In the oncogenic driver space, next generation sequencing has become a crucial tool for establishing an initial diagnosis and subsequently exploring resistance mechanisms induced by TKIs. The most common and arguably important resistance mechanism is development of the EGFR T790M mutation in approximately 60% of EGFR mutant NSCLC patients receiving a 1st generation EGFR TKI (18). The importance of identifying this mutation is due to the success of osimertinib which leads to improved survival outcomes in patients with T790M-positive EGFR TKI refractory disease and it has the added benefit of providing significant clinical responses in the CNS (19).
Leptomeningeal disease in the context of 1st generation EGFR TKI resistance is not uncommon and poses a challenging clinical conundrum. In keeping with the literature, we found that none of the four patients in our series who had experienced disease progression on a 1st generation TKI developed the EGFR T790M mutation in the CSF. Among these patients, we found that one of them had a T790M mutation on tissue biopsy and the patient experienced a short but clinically relevant CNS response with osimertinib. Two patients were treated with weekly-pulsed erlotinib with mixed results. The patient highlighted as the case study experienced a rapid and clinically meaningful benefit with osimertinib that remains durable 9 months later.
The clinical benefit of osimertinib in the absence of the EGFR T790M mutation supports the hypothesis that it is chiefly ‘pharmacokinetic failure’ of 1st generation EGFR TKIs in the CNS, with an inability to reach sufficient inhibitory concentrations in the CSF, that leads to disease progression. As there is little drug-induced selection pressure allowing the emergence of a resistant clone, the original EGFR driver mutation remains the most likely mutation profile found in the CSF. Hence, leptomeningeal disease is likely to be a phenomenon of reduced blood brain barrier TKI activity, allowing CNS progression whilst controlling extra-cranial disease, rather than a problem arising from resistance mutations. Thus, the development of the EGFR T790M mutation in CSF would be unlikely.
One way of better understanding the differential pharmacokinetic effects of EGFR TKIs in the plasma and CSF is to perform bioavailability studies and pharmacokinetic analysis as described by Planchard et al. (20). In brief, plasma and CSF will be collected both pre-dose and post-dose at set time-points up to 24 hours and analysed for osimertinib and two active metabolites, AZ5104 and AZ7550, simultaneously after protein precipitation, using a validated high performance liquid chromatography tandem mass spectrometry method. Pharmacokinetic analysis parameters would be performed by area under the plasma-concentration time curve (AUC) and calculated using the linear up/log down method.
Although it has been postulated that double-dose osimertinib, such as that employed in the BLOOM study achieves satisfactory CSF inhibitory levels, our case study suggests that standard dose osimertinib leads to excellent CNS activity and a meaningful clinical response. Another group has also recently shown promising response rates with standard dose osimertinib in four patients with T790M-negative leptomeningeal disease (21). Therefore, until safety and efficacy results from the BLOOM cohort investigating 160 mg daily osimertinib is published, standard dose osimertinib is a reasonable option for these individuals.
Our series confirms the concept of spatiotemporal heterogeneity within individual patients with EGFR mutant NSCLC. In our series, we consistently found that CSF levels of the primary activating EGFR mutation was higher in CSF compared to plasma. This adds weight to the concept of pharmacokinetic failure in the CNS, as compared to systemic progression on 1st generation TKIs. Another important finding is the feasibility of ddPCR for mutational profiling of cell-free DNA in the CSF. ddPCR has previously been shown to effectively identify EGFR sensitising mutations in plasma, with clinical utility in genotyping and quantitative monitoring of cell-free DNA in plasma (22). This approach, although not without the logistical challenges of repeated lumbar punctures, may be useful for early evaluation of patients who do not appear to be clinically improving or who are unable to undergo adequate imaging to evaluate treatment response, such as those with contra-indications to MRI with gadolinium contrast.
Another potential benefit of ddPCR and gene sequencing is investigating for the C797S mutation, an acquired tertiary EGFR mutation, which is the most common resistance mutation to osimertinib (23). Two groups, including ourselves recently described clinical response to the combination of osimertinib and gefitinib or erlotinib in osimertinib-refractory patients who had both the T790M mutation and C797S mutation in a trans-allelic conformation (17,24). Among the three patients in our series who developed leptomeningeal disease on osimertinib, we identified one patient with the C797S mutation in plasma, but not in CSF. As the patient had a cis-allelic conformation, we did not treat the patient with combination TKI therapy. The ability to identify the allelic conformation of the C797S mutation and T790M mutation is an important strategy at an attempt in providing a further treatment option for patients sequenced from 1st generation to 3rd generation TKIs.
Conclusions
Leptomeningeal disease is a devastating and difficult to treat complication of TKI resistant EGFR mutant NSCLC. The pattern of resistance to TKIs is spatiotemporal in nature and resistance to 1st generation TKIs in the CNS is likely due to an inability to sufficiently penetrate the blood brain barrier, rather than being caused by a resistance mutation. For this reason, osimertinib at standard dosing could be considered for these patients, even without an identifiable EGFR T790M mutation in the CSF. On the other hand, ddPCR and next generation sequencing may have utility in identifying the C797S mutation and its allelic conformation with the T790M mutation in osimertinib-refractory disease, with clinical implications. Prospective data is needed to better understand this especially as clonal evolution is a critical aspect of TKI failure.
Acknowledgments
None.
Footnote
Conflicts of Interest: S Arulananda: Honoraria – Astra Zeneca, Roche and Merck-Sharpe Dohme. P Mitchell: Advisory boards – Astra Zeneca, Boehringer-Ingelheim, BMS, MSD, Celgene. Honoraria – Roche, Merck KGa. T John: Advisory boards – Merck, BMS, Roche, Astra Zeneca, Pfizer. Honoraria – Astra Zeneca, BMS, Roche, Pfizer and Merck. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Austin Health Human Research Ethics Committee (No. H2012/04446).
References
- Goncalves PH, Peterson SL, Vigneau FD, et al. Risk of brain metastases in patients with nonmetastatic lung cancer: Analysis of the Metropolitan Detroit Surveillance, Epidemiology, and End Results (SEER) data. Cancer 2016;122:1921-7. [Crossref] [PubMed]
- Oechsle K, Lange-Brock V, Kruell A, et al. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol 2010;136:1729-35. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal Metastases in Patients with NSCLC with EGFR Mutations. J Thorac Oncol 2016;11:1962-9. [Crossref] [PubMed]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Togashi Y, Masago K, Fukudo M, et al. Efficacy of increased-dose erlotinib for central nervous system metastases in non-small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Chemother Pharmacol 2011;68:1089-92. [Crossref] [PubMed]
- Jackman DM, Cioffredi LA, Jacobs L, et al. A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget 2015;6:4527-36. [Crossref] [PubMed]
- How J, Mann J, Laczniak AN, et al. Pulsatile Erlotinib in EGFR-Positive Non-Small-Cell Lung Cancer Patients With Leptomeningeal and Brain Metastases: Review of the Literature. Clin Lung Cancer 2017;18:354-63. [Crossref] [PubMed]
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res 2010;16:5873-82. [Crossref] [PubMed]
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018;29:i10-i19. [Crossref] [PubMed]
- Hata A, Katakami N, Yoshioka H, et al. Spatiotemporal T790M Heterogeneity in Individual Patients with EGFR-Mutant Non-Small-Cell Lung Cancer after Acquired Resistance to EGFR-TKI. J Thorac Oncol 2015;10:1553-9. [Crossref] [PubMed]
- Sasaki S, Yoshioka Y, Ko R, et al. Diagnostic significance of cerebrospinal fluid EGFR mutation analysis for leptomeningeal metastasis in non-small-cell lung cancer patients harboring an active EGFR mutation following gefitinib therapy failure. Respir Investig 2016;54:14-9. [Crossref] [PubMed]
- Zhao J, Ye X, Xu Y, et al. EGFR mutation status of paired cerebrospinal fluid and plasma samples in EGFR mutant non-small cell lung cancer with leptomeningeal metastases. Cancer Chemother Pharmacol 2016;78:1305-10. [Crossref] [PubMed]
- Yang JCH, Cho BC, Kim D-W, et al. Osimertinib for patients (pts) with leptomeningeal metastases (LM) from EGFR-mutant non-small cell lung cancer (NSCLC): Updated results from the BLOOM study. J Clin Oncol 2017;35:abstr 2020.
- Ahn MJ, Kim DW, Cho BC, et al. Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases (BLOOM): a phase 1, open-label, dose-escalation and dose-expansion study. Lancet Respir Med 2017;5:891-902. [Crossref] [PubMed]
- Arulananda S, Do H, Musafer A, et al. Combination Osimertinib and Gefitinib in C797S and T790M EGFR-Mutated Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1728-32. [Crossref] [PubMed]
- Minari R, Bordi P, Tiseo M. Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in T790M-positive non-small cell lung cancer: review on emerged mechanisms of resistance. Transl Lung Cancer Res 2016;5:695-708. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Planchard D, Brown KH, Kim DW, et al. Osimertinib Western and Asian clinical pharmacokinetics in patients and healthy volunteers: implications for formulation, dose, and dosing frequency in pivotal clinical studies. Cancer Chemother Pharmacol 2016;77:767-76. [Crossref] [PubMed]
- Saboundji K, Auliac JB, Perol M, et al. Efficacy of Osimertinib in EGFR-Mutated Non-Small Cell Lung Cancer with Leptomeningeal Metastases Pretreated with EGFR-Tyrosine Kinase Inhibitors. Target Oncol 2018;13:501-7. [Crossref] [PubMed]
- Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res 2014;20:1698-705. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Wang Z, Yang JJ, Huang J, et al. Lung Adenocarcinoma Harboring EGFR T790M and In Trans C797S Responds to Combination Therapy of First- and Third-Generation EGFR TKIs and Shifts Allelic Configuration at Resistance. J Thorac Oncol 2017;12:1723-7. [Crossref] [PubMed]


