
The mSHOX2 is capable of assessing the therapeutic effect and predicting the prognosis of stage IV lung cancer
Introduction
Lung cancer ranks the highest in cancer morbidity and mortality in the world. Low-dose computed tomography (LDCT) has been recommended as the screening method for lung cancer (1). However, in scenarios that LDCT is not accessible, the in vitro diagnostic (IVD) methods may provide options for lung cancer early detection. The CE-approved Epi proLung is a recently developed assay for lung cancer early detection (2-14). Many other IVD methods for lung cancer screening or early detection, including those using the next-generation sequencing (NGS) technology and blood-based circulating tumor DNA (ctDNA) analysis, are currently under development and exhibit promising application perspectives (15-20). However, there is no effective IVD assay so far for therapeutic effect assessment or prognosis prediction in lung cancer. Clinically used protein markers, such as cyfra21-1, neuron-specific enolase (NSE), squamous cell carcinoma (SCC) and progastrin-releasing peptide (proGRP), are not appropriate for these applications, as their detection sensitivity is not satisfactory, and patients with negative results before therapy cannot be assessed after therapy. Furthermore, they are more sensitive to late stage lung cancer than early stage lung cancer, and are used more frequently as a marker for recurrence monitoring than therapeutic effect monitoring. The computed tomography (CT) is another non-invasive method for therapeutic effect assessment. However, CT cannot be used routinely as a monitoring examination, as the radiation method cannot be repeated frequently as an instant test. Therefore, it is lack of effective way for frequent therapeutic effect monitoring and prognosis prediction during and following lung cancer therapy.
The mSHOX2 assay is the first blood-based test recently developed as a lung cancer screening test. It has been proved as a sensitive and specific assay for blood-based lung cancer early detection (4,10,11). The assay detects abnormally methylated SHOX2 gene from ctDNA. Studies have found that the detection sensitivity of the mSHOX2 test was positively correlated with the severity of lung cancer (2,4), suggesting that the plasma mSHOX2 level could be an indicator for disease progression or relief. However, the observation on blood mSHOX2 level change following therapy is very limited (21). Since it was found that the level of blood methylation markers can be used as indicators for therapeutic effect assessment and prognosis prediction, we would like to investigate the potentials of blood mSHOX2 in these applications.
In the present study, we tested whether blood mSHOX2 is capable of assessing the therapeutic effect and predicting the long-term prognosis of stage IV lung cancer patients undergoing first-line standard chemotherapy, combined radio- and chemotherapy or tyrosine kinase inhibitor (TKI)-based targeted therapy. By collecting the blood samples from lung cancer patients before therapy and two cycles after therapy, we investigated the relationship between the blood mSHOX2 level change and the degree of patient response. The mSHOX2 level change exhibited linear correlation with tumor size change, facilitating its use in assessment and monitoring. The blood mSHOX2 levels before and after two cycles of therapy were also predictors for patient long-term survival. Our study provided strong evidence for the use of mSHOX2 in the therapeutic effect assessment and prognosis prediction of stage IV lung cancer patients.
Methods
Ethics
The permission for clinical study was granted by the ethics committees of all participating hospitals before the start of sample collection. Informed consent was obtained from all subjects, and the information on the usage of plasma and test results were provided to all subjects.
Study design, patients, and therapy
The study was designed and implemented in four Chinese hospitals using the mSHOX2 test in Epi proLung assay (Epigenomics AG, Berlin, Germany). Clinical status was determined before blood draw for mSHOX2 assay, and blood samples were obtained from all subjects who met the selection criteria. All technicians were blinded to the clinical information of subjects. A total of 163 subjects were recruited in this study, including 30 stage I, 29 stage II, 26 stage III and 68 stage IV lung cancer patients (Table 1). The staging was based on the National Comprehensive Cancer Network (NCCN) guidelines on cancer stage (22). Determination of stage was based on pathological examinations of biopsies from needle aspiration or surgery. First-line standard chemotherapy, combined radio- and chemotherapy, or TKI-based targeted therapy was performed for stage IV patients. The primary tumors were the target tumors in this study, and the diameter of the primary tumors were assessed based on RECIST 1.1 criteria, and correlated with the level of mSHOX2.The assessment of non-target lesions, lymph node metastasis, distal metastasis or new lesions was not included in this study.

Full table
Sample size estimation
The equation for known positive detection rate was used for sample size estimation: N=Z2*[p*(1−p)]/E2. Z is a statistical parameter (Z =1.96 for 95% CI) and E represented the error (10% was chosen in this study), and p represented the putative positive detection rate. The p value represented the known sensitivity for Epi proLung assay on lung cancer, and was obtained from a previous pilot study. If the known sensitivity for stage IV lung cancer equals to 0.90, an estimated 35 lung cancer cases were required. The study goal was to recruit 58 patients, anticipating a 40% loss of follow-up rate (Table 1). Stage IV lung cancer involved 24 small cell lung cancer (SCLC) and 44 non-small cell lung cancer (NSCLC) patients, and NSCLC involved 16 patients with EGFR-sensitive mutations (EGFR M+) underwent TKI-based first-line therapy and 28 patients without EGFR-sensitive mutations (EGFR M−) underwent standard chemotherapy or combined chemo- and radiotherapy.
Sample collection and storage
Blood samples were collected before the start of therapy and two cycles after the therapy. One cycle of chemotherapy lasted for 21 days, while patients for TKI therapy took medicine every day. The blood collection point after therapy was therefore set at day 42 for all patients, just before the start of the third cycle of chemotherapy. A 10 mL peripheral blood sample was collected with a 10 mL K2EDTA anticoagulant tube (BD biosciences, Franklin Lakes, NJ, USA). Sample storage and transportation followed the instructions for use of the Epi proLung assay. The sample information was recorded in sample collection forms. Plasma samples from all participating hospitals were prepared in individual hospitals and stored under −20 °C before they were delivered to Beijing BioChain Medical Laboratory, and all assays were performed in the same laboratory within three weeks from the sample collection date. The sample quality was examined when the samples arrived at the medical laboratory. Samples with plasma volume less than 3.5 mL, or with apparent hemolysis, high bilirubin, chylemia, or visible particles or pellets were not tested, and repeated blood draw was requested.
DNA extraction and qualitative PCR analysis of SHOX2
DNA extraction and bisulfite conversion were performed manually following the manufacturer’s instructions of Epi proLung assay. The bisDNA (bisulfite-converted DNA before methylation analysis) was assayed on an ABI7500 Fast Dx Real Time PCR device (Life Technologies) with Epi proLung kits. The PCR assay was modified by Beijing BioChain Medical Laboratory to examine the level of mSHOX2 alone, instead of both mSHOX2 and mPTGER4. The reaction condition and PCR parameters remained the same as the Epi proLung kits. PCR was performed in triplicate with 15 µL template DNA per well and run for 45 cycles. The validity of each sample batch was determined on the basis of methylated SHOX2 and ACTB threshold count (Ct) values for the positive and negative controls. ACTB was used as an internal reference to assess the integrity of each sample.
Data analysis and interpretation
Test data of the Epi proLung assay were analyzed by calculating the ΔΔCt values using the Ct values from samples, ACTB internal controls and the positive controls. Statistical analysis was performed and figures were plotted with GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA 92037, USA). For each sample, a relative methylation value was determined using the ΔΔCt method adapted for DNA methylation analyses as previously described (6). In brief, ΔΔCt values were calculated as below:

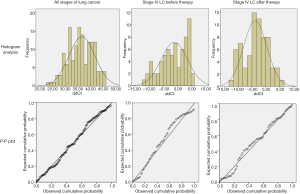
In order to identify the distribution pattern of mSHOX2 ΔΔCt values, histogram analysis, probability-probability (P-P) plot and the Kolmogorove-Smirnov (KS) test were performed (Figure S1). The mSHOX2 ΔΔCt values conformed to the normal distribution in all stages of lung cancer, stage IV lung cancer before therapy and stage IV lung cancer after therapy. The P values from the KS test for the above three groups were 0.473, 0.485 and 0.675, respectively (P>0.05 indicates no significant difference to normality). Therefore, student t-test was used in this study for significance analysis when comparing two groups.
Results
The plasma level of mSHOX2 was quantitatively correlated with tumor size in lung cancer
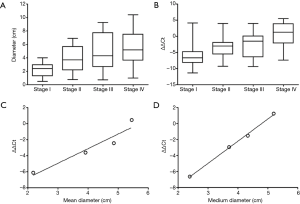
In order to discover the correlation between tumor size and the plasma mSHOX2 level, we first performed a validation study by looking at the relationship between lung cancer tumor size and the mSHOX2 ΔΔCt values. The relationship was shown in Figure 1 by studying the diameter and the mSHOX2 level in stage I to stage IV lung cancer. It appeared that the mSHOX2 level (Figure 1B) increased with the elevation of tumor stage and tumor size (Figure 1A). The relationship between the mean diameter or the median diameter for each stage and the ΔΔCt values was shown in Figure 1C,D, respectively. Good linear relationship was found between the tumor size and quantified mSHOX2 level (ΔΔCt values), with r2 at 0.92 and 0.99 for mean and median diameter, respectively. These results proved the linear correlation between lung cancer tumor size and the plasma mSHOX2 level.
The therapeutic effect of stage IV lung cancer can be quantitatively assessed by the plasma mSHOX2
The plasma mSHOX2 levels were then measured before and after the therapy of stage IV lung cancer patients to study whether the assay can be used to assess the therapeutic effect of these patients. Stage IV patients underwent first-line chemotherapy, combined radio- and chemotherapy or TKI-based targeted therapy (for subjects with EGFR-sensitive mutations) in this study. Blood draws were performed within one week before therapy and after two cycles of therapy (before the start of the third cycle), and therapeutic effect assessment was performed after two cycles of therapy (before the start of the third cycle) based on RECIST 1.1. Primary tumors were target tumors in the therapy and their maximal diameter was measured for the assessment.
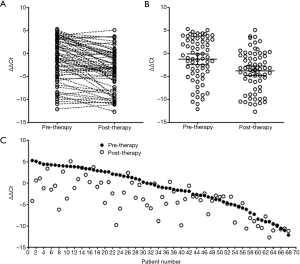
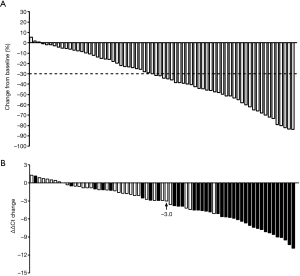
It can be observed from Figure 1A that in 68 stage IV patients recruited in the study, most patients exhibited mSHOX2 level decrease following two cycles of therapy, while some exhibited slightly increased or steady mSHOX2 level after therapy (Figure 2A). The mean ΔΔCt values decreased from −1.25 (95% CI: −2.39 to −0.10) to −3.78 (95% CI: −4.83 to −2.73) (Figure 2B), exhibiting an overall significant decrease of mSHOX2 level following two cycles of therapy (student t-test, P=0.0014). The mSHOX2 level change for each individual is clearly shown in Figure 2C, in which the patients were ranked from highest to lowest based on pre-therapeutic plasma mSHOX2 level. Although most patients showed decreased mSHOX2 level, the extent of decrease exhibited large variation among individuals.
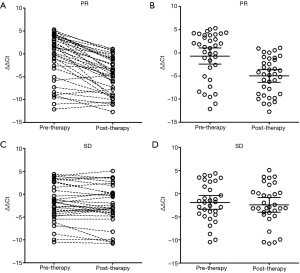
The details of the correlation between mSHOX2 level change and the therapeutic effect were further investigated by dividing the patients into two groups based on therapeutic response. In the partial response (PR) group (Figure 3A,B), it was very clear that most patients exhibited sharply decreased or steady mSHOX2 level after two cycles of therapy (Figure 3A), and the mean mSHOX2 level decreased from −0.68 (95% CI: −2.44 to 1.08) to −4.99 (95% CI: −6.34 to −3.64) (Figure 2B) (student t-test, P=0.0002). In contrast, the mean mSHOX2 level changed from −1.89 (95% CI: −3.38 to −0.40) to −2.42 (95% CI: −3.98 to −0.85) (Figure 2B) in the stable disease (SD) group, exhibiting essentially no change following therapy (student t-test, P=0.62). These observations clearly suggest that mSHOX2 level change was a sensitive indicator for therapeutic response, which was consistent with the assessment by computed tomography based on RECIST 1.1.
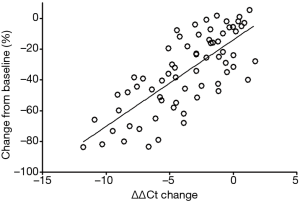
The therapeutic response and the corresponding mSHOX2 level change are shown in Figure 4A,B, respectively. Thirty-six patients out of 68 achieved PR while the rest 32 achieved SD following two cycle of therapy, regardless of the personalized strategies (Figure 4A). The mSHOX2 level (ΔΔCt) change was shown in descending order in Figure 4B, in which patients with PR (solid bars) generally exhibited bigger ΔΔCt change than those with SD (blank bars). The relationship between the response and the ΔΔCt change was plotted in Figure 6 and linear relationship can be obtained (r2=0.57). These results indicate that mSHOX2 level change was consistent with the response and was a good indicator for non-invasive therapeutic effect assessment.
The plasma mSHOX2 level predicted the long-term survival of stage IV lung cancer patients
Generally speaking, most stage IV lung cancer patients who receive first-line therapy will move to multiline therapy due to the development of resistance to first-line therapy. The multiline therapy include chemotherapy, combined chemo- and radiotherapy, TKI-based targeted therapy and immunotherapy. Here we would like to investigate the potential of mSHOX2 as a biomarker for early prediction of patient long-term prognosis before and at early-stage of the first-line therapy, no matter what type of multiline therapy was implemented.
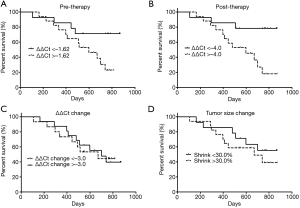
We therefore followed the patients for up to 871 days and collected the overall survival data from 31 patients available for follow-up. Figure 5 shows the overall survival data based on pre-therapy mSHOX2 level (Figure 5A), two cycles post-therapy mSHOX2 level (Figure 5B), mSHOX2 level change (ΔΔCt change) (Figure 5C) or the primary tumor size change (Figure 5D). When using the median as the subgrouping threshold, patients with low pre-therapeutic mSHOX2 level (i.e., ΔΔCt <−1.62) exhibited significantly better survival rate than those with high pre-therapeutic mSHOX2 level (i.e., ΔΔCt >−1.62) (Figure 5A, P=0.04). The median survival for patients with high pre-therapeutic mSHOX2 level was 598 days, while the median survival for patients with low pre-therapeutic mSHOX2 level has not been reached. Similarly, patients with low post-therapeutic mSHOX2 level (i.e., ΔΔCt <−4.0) exhibited significantly better survival rate than those with high post-therapeutic mSHOX2 level (i.e., ΔΔCt >−4.0) (Figure 5B, P=0.008). The median survival for patients with high post-therapeutic mSHOX2 level was 598 days, while the median survival for patients with low post-therapeutic mSHOX2 level has not been reached. In contrast, neither the ΔΔCt change (Figure 5C, P=0.66) nor the tumor size change (Figure 5D, P=0.40) showed significant survival difference, when the median was used as the subgrouping threshold. Univariable and multivariable Cox regression analysis [Forward: likelihood ratio (LR)] revealed that mSHOX2 level before therapy was the only independent predictor of the overall survival (P=0.032), with a hazard ratio of 1.414. These observations suggest that the pre-therapeutic plasma mSHOX2 level was a predicting factor for patient long-term survival, while the mSHOX2 level change or tumor size change before and after therapy were not predicting factors.
Discussion
The dynamic alterations of the blood mSHOX2 level following first-line therapy of stage IV lung cancer
The relationship between the level of plasma methylation markers (including mSHOX2, mSEPT9 and mPTGER4) and the progression of has been reported in previous studies of lung cancer and colorectal cancer. Generally, the detection rate and the plasma methylation level increase with the elevation of cancer stage, and good correlation was found between tumor size and plasma methylation level (2,4,11,23-25). It has been reported that methylation level of mSHOX2, mSEPT9, mPTGER4 and mPITX2 before or after therapy could be used as predicting markers for patient survival in colorectal cancer, lung cancer, neck squamous cell cancer, and gliomas (6,21,26-30). However, these markers were previous developed as diagnostic markers, and most of the studies focused on relatively early-stage cancers, instead of advanced or late-stage cancers. With the rapid development of targeted therapy and immunotherapy, stage IV lung cancer can be treated, the primary and metastatic cancers can be controlled, and therefore some patients can survive a long time. Our study focused on the monitoring and assessment of these patients and tried to identify a strategy to evaluate the therapeutic effect and predict the long-term outcome of various therapies.
It was obvious in our study and previous studies that mSHOX2 can be used for monitoring the progression, relief or response to therapy. This assessment should not be dependent on a single test, but a series of continuous tests at key assessment time points. Dynamic alterations of mSHOX2 levels are indicative for treatment response. Plasma mSHOX2 can therefore be used as non-invasive monitoring method for instant therapeutic effect assessment, and can be more safely and frequently used than CT. Although methylation markers have been shown to be sensitive for assessment, they are still not applicable in therapeutic strategy selection, in which mutation detection using NGS technology are currently widely used. Combination of mutation detection and methylation detection may be a good choice for both strategy selection and response monitoring, as methylation detection can also be achieved by NGS.
We found that a large majority of patients exhibited parallel response between tumor shrinkage and mSHOX2 level decrease (Figures 2-4), which achieved the linear relationship between them (Figure 6). However, the correlation between plasma mSHOX2 decrease and maximal diameter reduction for each individual is still hard to predict. This may be due to the way of measurement of tumor size and the mSHOX2 level. The measurement of maximal diameter at the same CT section may not fully reflect the tumor size change, while the mSHOX2 quantification for low level of mSHOX2 may not be very accurate. Ideally, the volume of tumor can be assessed by 3D reconstruction, and the mSHOX2 level can be quantified by digital PCR.
The application of mSHOX2 assay in predicting the long-term prognosis of metastatic lung cancer
It was interesting in this study that the mSHOX2 level before and two cycles after therapy were predictive for patient survival, while the mSHOX2 level change or tumor diameter change were not predictive. This suggests that the initial mSHOX2 level before therapy actually predicted long-term survival, and patients with relatively higher pre-therapeutic mSHOX2 level appeared to live shorter, no matter what types of therapy they receive in the following treatment. The earliest predicting time can be the time that lung cancer is diagnosed before any therapy. This suggests that mSHOX2 predicts the therapeutic response instead of merely reflecting the clinical course. The mSHOX2 level after two cycles of therapy was also predictive for survival, and this may be relevant to both pre-therapeutic mSHOX2 level and the degree of mSHOX2 level change following therapy. However, the fact that mSHOX2 level change and tumor diameter change were not predictive suggests that the initial response to first-line therapy did not predict the long-term survival. Patients with initial good response to first-line therapy may not have good prognosis in future.
It was found that the mSHOX2 level before therapy had huge variation (Figure 2A). Since the pre-therapeutic mSHOX2 level appeared to be predictive for long-term survival, it would be interesting to know the reason for this variation and the decisive factors for mSHOX2 level. Generally speaking, the plasma level of methylation markers may be relevant to tumor size, tumor growth pattern, and tumor pathological type. We previous found in colorectal cancer that patients with moderate to low differentiation exhibited significantly higher plasma mSEPT9 level than those with high differentiation. Patients with infiltrative CRC exhibited significantly higher plasma mSEPT9 level than those with protrude or ulcerative CRC. Patients with N2 lymph node metastasis exhibited significantly higher plasma mSEPT9 level than those with N1 lymph node metastasis or no lymph node metastasis. Patients with distal metastasis exhibited significantly higher plasma mSEPT9 level than those with no metastasis (31). Although no such studies have been performed with lung cancer, it can be speculated that similar factors in lung cancer may affect the plasma mSHOX2 level, which, in turn, affect the long-term survival.
Opportunities and challenges of methylation-based assay in future lung cancer therapeutic effect assessment and prognosis prediction
The plasma single gene-based methylation assay has already exhibited promising clinical perspectives. mSHOX2 and a couple of other methylation markers have been shown to be effective for therapeutic effect assessment and prognosis prediction. However, it is still difficult to predict the individual risk or prognosis based on methylation markers. The individual prediction may be composed of many factors other than methylation biomarkers, and a quantified scoring system might be used to provide a definite interpretation. Another approach is to build a panel-based methylation model to assess the therapeutic effect and to predict the prognosis. This may overcome the deficiency of a single marker and make the assessment and prediction more accurate. However, this needs validation and optimization of a NGS-based methylation panel, and algorithm, modeling or even artificial intelligence may be required for prediction.
It would also be interesting if methylation markers can be used in patient selection for specific therapy, similar to the EGFR-sensitive mutations for selecting patients for TKI therapy or tumor mutation burden (TMB) assay for immunotherapy patient selection. This may be applied to some new therapies or drugs relevant to patient methylation status, or some current therapies in which patients can be selected by methylation. Methylation may be the next revolutionary marker for cancer therapy if it can be used in patient selection. Furthermore, recurrence could be predicted by circulating methylation markers in future, as there is evidence showing that the mutation detection by NGS from ctNA can predict the recurrence of lung cancer before signs from imaging tests can be identified. However, evidence is not available for mSHOX2 and more investigation is needed.
Conclusions
Quantification of the plasma mSHOX2 is capable of monitoring and assessing the therapeutic effect of stage IV lung cancer patients undergoing chemotherapy, combined chemo- and radiotherapy, or targeted therapy. Both pre-therapeutic and post-therapeutic plasma mSHOX2 quantification are effective for patient long-term prognosis prediction.
Future perspective
Individualized risk assessment, therapeutic effect assessment and prognosis prediction by methylation markers would be one of the future directions for blood-based methylation assays. This may require combination of multiple markers with clinical characteristics to achieve model prediction. Methylation panel assay by NGS may be an option for multiple marker detection, and can be applied to all cancers other than lung cancer. Selection of therapeutic strategies based on methylation status might be another future direction with huge potential.
Acknowledgments
Funding: This study was funded by the Beijing Natural Science Foundation Project (No. 7152143), sponsored by the Beijing Natural Science Foundation Committee, and the Beijing Municipal Science and Technology Project (capital public health project) (No. Z151100003915092), sponsored by the Beijing Municipal Science and Technology Commission, and the Postdoctoral Science Foundation of China (No. 201003778).
Footnote
Conflicts of Interest: L Song was previously an employee of BioChain (Beijing) Science and Technology, Inc. BioChain is a collaborator of Epigenomics AG, a Germany-based company that launched the first commercial mSHOX2 assay. The other authors have no conflicts of interest to declare.
Ethical Statement: The permission for clinical study was granted by the ethics committees of all participating hospitals before the start of sample collection. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all subjects, and the information on the usage of plasma and test results were provided to all subjects.
References
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Schmidt B, Liebenberg V, Dietrich D, et al. SHOX2 DNA methylation is a biomarker for the diagnosis of lung cancer based on bronchial aspirates. BMC Cancer 2010;10:600. [Crossref] [PubMed]
- Schneider KU, Dietrich D, Fleischhacker M, et al. Correlation of SHOX2 gene amplification and DNA methylation in lung cancer tumors. BMC Cancer 2011;11:102. [Crossref] [PubMed]
- Kneip C, Schmidt B, Seegebarth A, et al. SHOX2 DNA methylation is a biomarker for the diagnosis of lung cancer in plasma. J Thorac Oncol 2011;6:1632-8. [Crossref] [PubMed]
- Dietrich D, Kneip C, Raji O, et al. Performance evaluation of the DNA methylation biomarker SHOX2 for the aid in diagnosis of lung cancer based on the analysis of bronchial aspirates. Int J Oncol 2012;40:825-32. [PubMed]
- Dietrich D, Hasinger O, Liebenberg V, et al. DNA methylation of the homeobox genes PITX2 and SHOX2 predicts outcome in non-small-cell lung cancer patients. Diagn. Mol Pathol 2012;21:93-104. [Crossref] [PubMed]
- Darwiche K, Zarogoulidis P, Baehner K, et al. Assessment of SHOX2 methylation in EBUS-TBNA specimen improves accuracy in lung cancer staging. Ann Oncol 2013;24:2866-70. [Crossref] [PubMed]
- Ilse P, Biesterfeld S, Pomjanski N, et al. SHOX2 DNA methylation is a tumour marker in pleural effusions. Cancer Genomics Proteomics 2013;10:217-23. [PubMed]
- Ilse P, Biesterfeld S, Pomjanski N, et al. Analysis of SHOX2 methylation as an aid to cytology in lung cancer diagnosis. Cancer Genomics Proteomics 2014;11:251-8. [PubMed]
- Konecny M, Markus J, Waczulikova I, et al. The value of SHOX2 methylation test in peripheral blood samples used for the differential diagnosis of lung cancer and other lung disorders. Neoplasma 2016;63:246-53. [PubMed]
- Weiss G, Schlegel A, Kottwitz D, et al. Validation of the SHOX2/PTGER4 DNA Methylation Marker Panel for Plasma-Based Discrimination between Patients with Malignant and Nonmalignant Lung Disease. J Thorac Oncol 2017;12:77-84. [Crossref] [PubMed]
- Ren M, Wang C, Sheng D, et al. Methylation analysis of SHOX2 and RASSF1A in bronchoalveolar lavage fluid for early lung cancer diagnosis. Ann Diagn Pathol 2017;27:57-61. [Crossref] [PubMed]
- Ni S, Ye M, Huang T. Short stature homeobox 2 methylation as a potential noninvasive biomarker in bronchial aspirates for lung cancer diagnosis. Oncotarget 2017;8:61253-63. [Crossref] [PubMed]
- Zhang C, Yu W, Wang L, et al. DNA Methylation Analysis of the SHOX2 and RASSF1A Panel in Bronchoalveolar Lavage Fluid for Lung Cancer Diagnosis. J Cancer 2017;8:3585-3591. [Crossref] [PubMed]
- Belloni E, Veronesi G, Rotta L, et al. Whole exome sequencing identifies driver mutations in asymptomatic computed tomography-detected lung cancers with normal karyotype. Cancer Genet 2015;208:152-5. [Crossref] [PubMed]
- Uchida J, Kato K, Kukita Y, et al. Diagnostic Accuracy of Noninvasive Genotyping of EGFR in Lung Cancer Patients by Deep Sequencing of Plasma Cell-Free DNA. Clin Chem 2015;61:1191-6. [Crossref] [PubMed]
- Jin X, Chen Y, Chen H, et al. Evaluation of Tumor-Derived Exosomal miRNA as Potential Diagnostic Biomarkers for Early-Stage Non-Small Cell Lung Cancer Using Next-Generation Sequencing. Clin Cancer Res 2017;23:5311-9. [Crossref] [PubMed]
- Leng Q, Lin Y, Jiang F, et al. A plasma miRNA signature for lung cancer early detection. Oncotarget 2017;8:111902-11. [Crossref] [PubMed]
- Ye M, Li S, Huang W, et al. Comprehensive targeted super-deep next generation sequencing enhances differential diagnosis of solitary pulmonary nodules. J Thorac Dis 2018;10:S820-S829. [Crossref] [PubMed]
- Feng Y, Feng G, Lu X, et al. Exploratory analysis of introducing next-generation sequencing-based method to treatment-naive lung cancer patients. J Thorac Dis 2018;10:5904-12. [Crossref] [PubMed]
- Schmidt B, Beyer J, Dietrich D, et al. Quantification of cell-free mSHOX2 Plasma DNA for therapy monitoring in advanced stage non-small cell (NSCLC) and small-cell lung cancer (SCLC) patients. PLoS One 2015;10:e0118195. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Powrózek T, Krawczyk P, Kucharczyk T, et al. Septin 9 promoter region methylation in free circulating DNA-potential role in noninvasive diagnosis of lung cancer: preliminary report. Med Oncol 2014;31:917. [Crossref] [PubMed]
- Behrouz Sharif S, Hashemzadeh S, Mousavi Ardehaie R, et al. Detection of aberrant methylated SEPT9 and NTRK3 genes in sporadic colorectal cancer patients as a potential diagnostic biomarker. Oncol Lett 2016;12:5335-43. [Crossref] [PubMed]
- Song L, Jia J, Peng X, et al. The performance of the SEPT9 gene methylation assay and a comparison with other CRC screening tests: A meta-analysis. Sci Rep 2017;7:3032. [Crossref] [PubMed]
- Zhang YA, Zhou Y, Luo X, et al. SHOX2 is a Potent Independent Biomarker to Predict Survival of WHO Grade II-III Diffuse Gliomas. EBioMedicine 2016;13:80-9. [Crossref] [PubMed]
- Dietrich D, Jung M, Puetzer S, et al. Diagnostic and prognostic value of SHOX2 and SEPT9 DNA methylation and cytology in benign, paramalignant and malignant pleural effusions. PLoS One 2013;8:e84225. [Crossref] [PubMed]
- Jung M, Pützer S, Gevensleben H, et al. Diagnostic and prognostic value of SHOX2 and SEPT9 DNA methylation and cytology in benign, paramalignant, and malignant ascites. Clin Epigenetics 2016;8:24. [Crossref] [PubMed]
- Schröck A, Leisse A, de Vos L, et al. Free-Circulating Methylated DNA in Blood for Diagnosis, Staging, Prognosis, and Monitoring of Head and Neck Squamous Cell Carcinoma Patients: An Observational Prospective Cohort Study. Clin Chem 2017;63:1288-96. [Crossref] [PubMed]
- de Vos L, Gevensleben H, Schröck A, et al. Comparison of quantification algorithms for circulating cell-free DNA methylation biomarkers in blood plasma from cancer patients. Clin Epigenetics 2017;9:125. [Crossref] [PubMed]
- Song L, Wang J, Wang H, et al. The quantitative profiling of blood mSEPT9 determines the detection performance on colorectal tumors. Epigenomics 2018;10:1569-83. [Crossref] [PubMed]


