Validation of the 8th edition of the TNM staging system in 3,950 patients with surgically resected non-small cell lung cancer
Introduction
Despite the recent advances in diagnosis and treatment, lung cancer is the most frequent cause of cancer-related deaths in both men and women worldwide (1). Accurate staging of lung cancer is essential for predicting prognosis and selecting appropriate treatment; as such, the TNM staging system for lung cancer was significantly modified in its 8th edition (2), which was authorized by the American Joint Committee on Cancer (AJCC) on January 1, 2018.
The 8th edition of the TNM system was developed on the basis of extensive investigations by the International Association for the Study of Lung Cancer (IASLC), including the analysis of an international database of 94,708 patients from 46 sites from 19 countries (3). However, the database has some limitations in that a majority of the patients (76.9%) were from two countries (Japan and Denmark) and that the database lacked information on tumor recurrence. In addition, the database consisted of dichotomized patients classified by different LN maps—Naruke-Japanese (4) or Mountain-Dresler modification of the American Thoracic Society (MD-ATS) (5)—and the corresponding analysis was performed without statistical correction (6). Therefore, the IASLC carried out an external validation using the National Cancer Database and showed that the 8th edition had similar discrimination ability (7). Nevertheless, a further robust external validation using a large independent data set from an institution with standardized protocols should be carried out.
As the largest tertiary referral center in Korea, Asan Medical Center has maintained a nearly 100% completion rate for postoperative follow-up and less than 1% surgical mortality in the last decade. In this study, we validated the 8th edition of the TNM staging system by using the prospectively collected lung cancer database from our institution, and compared the discrimination values of the 7th and the 8th editions with respect to the overall survival (OS) and recurrence-free survival (RFS).
Methods
Patients
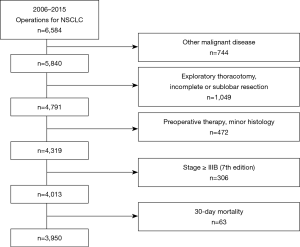
The clinical records of patients who underwent surgery for non-small cell lung cancer (NSCLC) were retrospectively collected between January 2006 and December 2015 in the thoracic surgery department of Asan Medical Center in Seoul, South Korea. Of these patients (n=6,584), the following patients were excluded: patients with other concurrent malignancies (n=744); patients who underwent sublobar resection (biopsy, wedge resection, and segmentectomy), incomplete resection (R1 and R2 resection) or incomplete lymph nodal dissection (number of resected lymph nodes <6) (n=1,049); patients who received preoperative chemoradiation therapy (n=201); patients who had histology other than adenocarcinoma, squamous cell carcinoma or adenosquamous cell carcinoma (n=271); patients whose stages were higher than IIIB according to the 7th edition (n=306) and/or who died within 30 days after surgery (n=63) (Figure 1). Consequently, 3,950 patients who underwent complete resection with systematic lymph node dissection were included. This study was approved by the Asan Medical Center Ethics Committee/Review Board (2019-0544).
The patients were pathologically staged according to the 8th edition in a retrospective manner (2). Adjuvant chemotherapy was used in patients with the 7th edition ≥ IIA and some with high-risk stage IB (lymphovascular invasion, visceral pleural involvement, large tumor size). Systemic chemotherapy with a platinum-based regimen was planned for four to six weeks after surgery, with a total of four courses of treatment. Follow-up information on all patients was obtained through clinic follow-up notes every 6 months during the first 5 years after surgery and every year thereafter. Chest CT scans were performed in sync with clinical visits or at any time when disease recurrence was clinically suspected. Treatment modalities and chemotherapeutic regimens in relapsed cases were determined at the discretion of the attending physician.
Statistical analysis
Continuous variables are presented as medians and interquartile ranges, and categorical variables as percentages. Survival curves of OS and RFS according to the 7th and the 8th editions were estimated using Kaplan-Meier survival analysis and assessed using the log-rank test. Cox proportional hazards model was used for univariate and multivariate analysis to identify prognostic factors of OS and RFS. Selection of the final multivariate model was processed with a stepwise model selection approach (P ≤0.1 for entering the model and P ≤0.05 for staying in the model). Cox proportional hazard analysis was also used to adjust for covariate variables and to calculate hazard ratios (HR) between adjacent TNM staging groupings. Before comparing the discrimination ability, we selected the final model for 7th and 8th editions by using multivariate analysis. Age, sex, and staging groupings were included in the final model for OS, whereas age, histology, history of adjuvant therapy, and staging groupings were included in the final model for RFS. The prognostic values of the two final multivariate models were calculated with the Akaike Information Criterion (AIC) (8) and the R2 measure (9), and the Concordance index (C-index) (10) was used in the two models to determine the discriminatory power.
All statistical calculations were performed using R version 3.2.5 (The R Foundation for Statistical Computing, Vanderbilt University, Nashville, TN, USA) using the Survival, ggplot2, GGally, and rms packages. P values less than 0.05 were considered significant.
Results
Patient characteristics
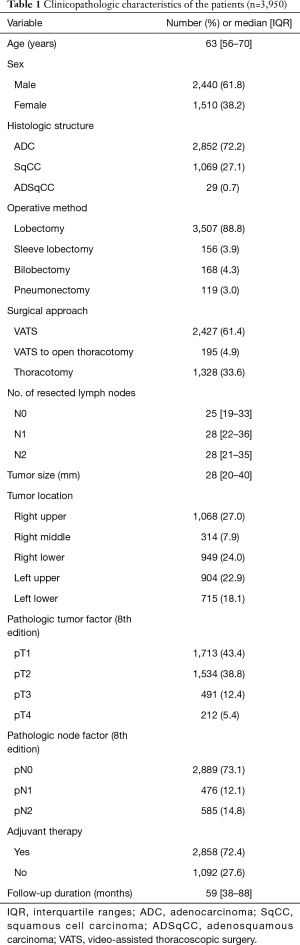
Clinicopathologic characteristics of the patients are summarized in Table 1. The median follow-up period was 59 months (interquartile range, 38–88 months). Lobectomy was performed in 3,663 patients (92.7%), and video-assisted thoracoscopic surgery was performed in 2,427 patients (61.4%). A total of 2,858 (72.4%) patients received adjuvant therapy, including chemotherapy, radiotherapy, or concurrent chemoradiation therapy.
Full table
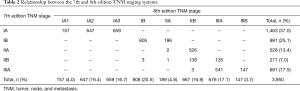
Distribution of patients following the 7th and 8th editions and the association between the two editions is shown in Table 2. After applying the 8th edition, patients in the 7th edition stage IA (n=1,463) were subdivided into 8th edition stages IA1 (n=157, 10.7%), IA2 (n=647, 44.2%), and IA3 (n=659, 45.0%), and a portion of the 7th edition stage IB patients were moved to the 8th edition stage IIA (186/991, 18.8%). Due to the change of T1N1 and T2aN1 patients from the 7th edition stage IIA to the 8th edition stage IIB, almost all patients in the 7th edition stage IIA were restaged to the 8th edition stage IIB (526/528, 99.6%). Almost all patients in the 7th edition stage IIB (n=277) were divided into the 8th edition stages IIB (n=138, 49.8%) and IIIA (n=135, 48.7%). In the 7th edition stage IIIA group, 21.3% (147/691) were moved to the 8th edition stage IIIB.
Full table
Analysis of OS and RFS
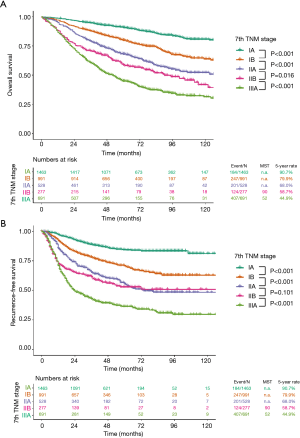
Survival curves of OS and RFS following the 7th and 8th editions are shown, along with median survival time and 5-year survival rates (Figures 2,3). The OS curves stratified by the 7th edition showed a stepwise deterioration from stage IA to stage IIIA (Figure 2A). A phased degradation was also found within the RFS curves, except that the curves of stage IIA and IIB were not significantly different (P=0.101) (Figure 2B). According to the 8th edition, survival curves of OS and RFS displayed sequential deteriorations, but did not show significant differences between stages IIA and IIB (P=0.172 for OS and P=0.144 for RFS) (Figure 3).
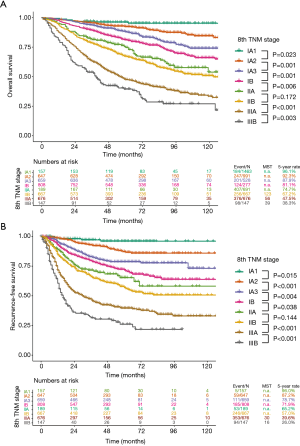
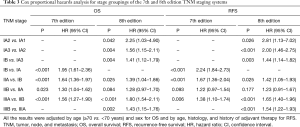
According to Cox proportional hazard analysis, all HRs between adjacent staging groups were higher than 1.0, indicating gradual deterioration of prognosis according to the staging groups (Table 3). However, a significant difference was not observed between stages IIA and IIB in the 8th edition for OS and in both the 7th and the 8th editions for RFS, which is in line with the results from the Kaplan-Meier analysis.
Full table
As shown in Table 4, the 8th edition had better model fit as indicated by the smaller AIC values (17,517 vs. 17,543 for OS, 16,720 vs. 16,784 for RFS) and better prediction accuracy as indicated by the higher R2 values (0.178 vs. 0.171 for OS, 0.158 vs. 0.143 for RFS). The 8th edition also showed better discriminatory ability as indicated by the higher C-index scores (0.753 vs. 0.751 for OS, 0.718 vs. 0.716 for RFS).

Full table
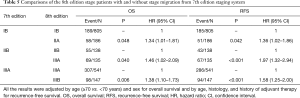
To evaluate the effectiveness of the modifications in the 8th edition, we compared the survival outcomes of the 7th edition stage IB, IIB, and IIIA divided by the 8th edition (Table 5). Using Cox regression models, we found significant differences of the 7th edition stage IB (P=0.048 for OS and P=0.042 for RFS), IIB (P=0.040 for OS and P<0.001 for RFS), and IIIA (P=0.006 for OS and P<0.001 for RFS), depending on whether or not stage migration was present, regardless of OS and RFS.
Full table
Discussion
The TNM staging system for malignant tumors is a globally accepted protocol for classifying the degree of tumor extent and invasion, thereby providing accurate prognosis and suggesting the appropriate treatment stratification (11). Several editions of the TNM staging system for lung cancer have been published following its introduction in 1973 (12); the latest update in 2017 introduced the 8th edition for lung cancer (2), which provided several new categories, especially in the T and M descriptors. As for the T descriptor, T1 and T2 were subdivided into T1a, T1b, T1c, T2a, and T2b by size in 1-cm increments. In addition, tumors larger than 5 and 7 cm were reclassified as T3 and T4, respectively. Tumors causing partial or total lung atelectasis and those involving main stem bronchus regardless of distance from the carina were reclassified as T2. Diaphragm invasion was reclassified as T4 and mediastinal pleural invasion was removed from the T descriptor (13). As for the M descriptor, tumors with extrathoracic metastases were subdivided into M1b involving single distant sites and M1c involving multiple distant sites (14). No changes were recommended for the N descriptor (6). In the new stage grouping, significant changes included subdivision of stage IA into IA1 (T1aN0), IA2 (T1bN0), and IA3 (T1cN0), and introduction of the new IIIC stage representing T3N3 and T4N3 tumors (15).
In our study, the curves of OS and RFS for each stage grouping showed a gradual deterioration (Figures 2,3). However, in both the 7th and 8th editions, there were no significant differences between the IIA and IIB groups, which remained the same on Cox analysis adjusted with multiple covariates (Table 3). These findings are in line with those from previous studies that validated the 7th edition (16,17) and the 8th edition (18). It is obvious that the absolute values of difference between stage IIA and IIB are not as large as those between other groups. However, relatively low proportions of patients in the 7th edition stage IIB (277/3,950, 7.0%) and the 8th edition stage IIA (189/3,950, 4.8%) also affected these results. In terms of the 7th edition, a small proportion of IIB patients was consistently reported in other external validation studies after the introduction of the 7th stage system (16,17). According to the definition of the 8th edition, almost all patients in the 7th edition stage IIA were restaged to 8th edition stage IIB; moreover, the percentage of stage IIA patients whose tumor size ranged from 4 to 5 cm without lymph node invasion was reduced to 4.8%. Therefore, comparison between the groups with a relatively small difference and a low percentage of patients may be responsible for the insignificant P values.
In our current study, we conducted a retrospective analysis in a large cohort from a single institution in order to determine whether the newly introduced 8th edition of the TNM classification system for NSCLC is a better prognosticator of OS and RFS than the previous 7th edition. Judging from the results adjusted through multivariate Cox analysis, the 8th edition seems to have better prognostic power (higher C-index) for OS and RFS. Considering that the discriminative value becomes higher as the number of prediction variables in a certain model increases (19), this result may be attributed to the subdivision of 7th edition stage IA tumors into 8th edition stages IA1, IA2, and IA3. However, even after applying the AIC and R2 methods that adjust their predictive value by penalizing the number of increased variables (20), the 8th edition still showed better prognostic ability (lower AIC and higher R2). Consequently, subdividing the 7th edition stage IA patients into three groups (IA1, IA2, and IA3) is a statistically appropriate modification, and the 8th edition is superior in terms of stratification of prognosis for OS and RFS. Proper migration of stage in patients in the 7th edition stages IB, IIB, and IIIA may also contribute to improved discrimination ability of the 8th edition (Table 5).
It is crucial to comment on the clinical guidelines that would be revised as patients move to different stages after applying the 8th edition. For the 7th edition stage IB, patients with tumor size >4 cm are upstaged to the 8th edition stage IIA, which is related to the controversy as to whether the 7th edition stage IB patients with high risk can benefit from adjuvant chemotherapy. Although no definitive agreement has been reached through randomized clinical trials (21,22), there is a consensus that adjuvant chemotherapy is helpful in the 7th edition stage IB patients with tumors >4 cm; as such, the National Comprehensive Cancer Network recommends them as candidates for adjuvant chemotherapy (23), which is reflected in the 8th edition by stage migration. On the other hand, multiple studies reported that patients with visceral pleura invasion (VPI) have significantly worse prognosis within the 7th edition stage IB and proposed them to be upstaged as stage IIA (24-26). However, these patients remain unchanged in the 8th edition. In our study, there was no significant difference of prognosis depending on whether VPI was present (339/727) or not (388/727) among the 8th edition stage IB patients without adjuvant chemotherapy, regardless of OS [HR 0.91, 95% confidence interval (CI): 0.57–1.46, P=0.384] and RFS [HR 1.25, 95% CI: 0.92–1.70, P=0.157]. However, this should be interpreted with caution because there may have been selection bias, considering the exclusion of VPI-positive patients who had more malignant potential (i.e., larger tumor size) and received adjuvant chemotherapy. In fact, among stage IB patients without adjuvant chemotherapy, VPI-positive patients had smaller tumor size than VPI-negative patients (26.6±7.6 vs. 33.1±6.4, P<0.001). Consequently, the prognostic effect of VPI is difficult to identify in our study.
Our study utilized a large independent database, and none of the patients were included in the IASLC database. However, our results are limited in generalizability due to the retrospective nature of the study design and the use of observational data from a single institution. Also, because all reviewed data were collected only from patients undergoing surgical treatment, the survival curves might not reflect those of the general NSCLC patient population.
Conclusions
We demonstrate that stratification according to the 8th edition of the TNM staging system is prognostically valid for patients who underwent complete resection of NSCLC. The discrimination ability of the 8th edition was superior to the 7th edition in terms of OS and RFS.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Asan Medical Center Ethics Committee/Review Board (2019-0544) and written informed consent was obtained from all patients.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-24.
- Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg 1978;76:832-9. [PubMed]
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [Crossref] [PubMed]
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2017;12:1109-21.
- Choi I, Wells BJ, Yu C, et al. An empirical approach to model selection through validation for censored survival data. J Biomed Inform 2011;44:595-606. [Crossref] [PubMed]
- Hielscher T, Zucknick M, Werft W, et al. On the prognostic value of survival models with application to gene expression signatures. Stat Med 2010;29:818-29. [Crossref] [PubMed]
- Uno H, Cai T, Pencina MJ, et al. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 2011;30:1105-17. [PubMed]
- Webber C, Gospodarowicz M, Sobin LH, et al. Improving the TNM classification: findings from a 10-year continuous literature review. Int J Cancer 2014;135:371-8. [Crossref] [PubMed]
- Motta G, Nahum MA, Testa T, et al. TNM staging system of lung carcinoma: historical notes, limitations and controversies. Ann Ital Chir 1995;66:425-32. [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Rena O, Massera F, Robustellini M, et al. Use of the proposals of the international association for the study of lung cancer in the forthcoming edition of lung cancer staging system to predict long-term prognosis of operated patients. Cancer J 2010;16:176-81. [Crossref] [PubMed]
- Kameyama K, Takahashi M, Ohata K, et al. Evaluation of the new TNM staging system proposed by the International Association for the Study of Lung Cancer at a single institution. J Thorac Cardiovasc Surg 2009;137:1180-4. [Crossref] [PubMed]
- Chen K, Chen H, Yang F, et al. Validation of the Eighth Edition of the TNM Staging System for Lung Cancer in 2043 Surgically Treated Patients With Non-small-cell Lung Cancer. Clin Lung Cancer 2017;18:e457-66.
- Hawkins DM. The problem of overfitting. J Chem Inf Comput Sci 2004;44:1-12. [Crossref] [PubMed]
- Miladinovic B, Kumar A, Mhaskar R, et al. A flexible alternative to the Cox proportional hazards model for assessing the prognostic accuracy of hospice patient survival. PLoS One 2012;7:e47804. [Crossref] [PubMed]
- Schmid-Bindert G, Engel-Riedel W, Reck M, et al. A randomized Phase 2 study of pemetrexed in combination with cisplatin or carboplatin as adjuvant chemotherapy in patients with completely resected stage IB or II Non-Small-Cell Lung Cancer. Lung Cancer 2015;90:397-404. [Crossref] [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Non-small cell lung cancer. 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Fibla JJ, Cassivi SD, Brunelli A, et al. Re-evaluation of the prognostic value of visceral pleura invasion in Stage IB non-small cell lung cancer using the prospective multicenter ACOSOG Z0030 trial data set. Lung Cancer 2012;78:259-62. [Crossref] [PubMed]
- Jiang L, Liang W, Shen J, et al. The impact of visceral pleural invasion in node-negative non-small cell lung cancer: a systematic review and meta-analysis. Chest 2015;148:903-11. [Crossref] [PubMed]
- Kawase A, Yoshida J, Miyaoka E, et al. Visceral pleural invasion classification in non-small-cell lung cancer in the 7th edition of the tumor, node, metastasis classification for lung cancer: validation analysis based on a large-scale nationwide database. J Thorac Oncol 2013;8:606-11.