Lipid management for coronary heart disease patients: an appraisal of updated international guidelines applying Appraisal of Guidelines for Research and Evaluation II—clinical practice guideline appraisal for lipid management in coronary heart disease
Introduction
Cardiovascular (CV) diseases are the leading cause of death worldwide (1-3). Among numerous CV risk factors, hyperlipidemia is one of the key factors for coronary heart diseases (CHD) and may even be a prerequisite for CHD (4-7). Therefore, the recommendations for lipid management are included in most CHD guidelines and there are even clinical practice guidelines (CPGs) for dyslipidemia published later. Guidelines like these usually provide recommendations based on abundant evidence as well as the most updated knowledge about best practices (8,9), and the ultimate purpose of them is to improve the outcome of CHD patients. However, clinicians may be confused about the various quality and recommendations of guidelines, as a result, the effectiveness of health care may be influenced. The Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument (10), updated in 2009, is the sole tool validated by an international group of researchers and recommended by World Health Organization. A systematic review of dyslipidemia CPGs published in 2015, found multiple things in common, such as intervention of therapeutic lifestyle, pharmacotherapy of statins, patients’ involvement and so on, together with many other differences (11). However, it did not include the widely referenced dyslipidemia CPGs published in recent years, and it neither considered CHD alone nor included relevant guidelines. Furthermore, due to the understanding gap between guidelines providers and clinicians, the implementation of guidelines in the daily clinical practice may be unsatisfactory (12,13). Thus, this paper attempts not only to use AGREE II instrument to appraise guidelines with lipid recommendations for CHD patients, but also to show clinicians the overall situation of major lipid recommendations in these guidelines.
Methods
To identify the appropriate guidelines with recommendations about lipid management for CHD patients, we conducted a systematic review on PubMed, Embase, Cochrane and official websites of CV guideline organizations and professional societies. The four following guideline databases were used to supplement our search: the National Guideline Clearinghouse (United States), the National Library for Health (United Kingdom) on Guideline Finder, Canadian Medical Association Infobase (Canada), and the Guideline International Network (G-I-N) International Guideline Library. We included both most updated CHD guidelines and dyslipidemia guidelines published from January 1, 2009, to January 1, 2019. The guidelines were confined in English and required free full-text.
Relative records were firstly screened on the title and abstract. Full-text was reviewed for identifying trustworthy CPGs. The Institute of Medicine defines CPGs as “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances”. The following aspects also need to be considered as trustworthy CPGs: evidence reviewing, a panel of experts, patients’ preferences, transparency of interest conflicts and the logicality between care options and health outcomes with evidence (14). Guidelines developed by local, regional, national or international groups or affiliated governmental organizations and recently updated were included. Guidelines were excluded with no information about lipid management for CHD patients. We also strictly selected articles published as a guideline instead of as a consensus or statement.
AGREE II instrument is a 23-item tool comprising 6 quality domains: (I) scope and purpose; (II) stakeholder involvement; (III) rigor of development; (IV) clarity of presentation; (V) applicability; (VI) editorial independence. Two reviewers (Zhang and X Zhuang) trained with research methods independently appraised the quality of guidelines by using the AGREE II tool. Each item was independently rated on the 1-7 points scale by 2 reviewers. Domain scores were calculated as standardized scores by the following formula: (obtained score − minimum possible score)/(maximum possible score − minimum possible score) (AGREE II) If the total score of 2 reviewers differed more than 20%, a third independent reviewer (X Sun) assessed the guideline. Final rigor scores were calculated by averaging the AGREE II scores from all reviewers. Radar graphs were applied to show the score results intuitively. A guideline was “strongly recommended” that if most domains (4 or more) scored above 60% and a guideline was “recommended with some modification” if most domains scored between 30% and 60% (15,16).
One reviewer (S Zhang) extracted all the basic information and related recommendations from each included guideline. The other reviewer (X Zhuang) checked the results for accuracy and completeness, disagreements between the reviewers were discussed and resolved by consensus. The characteristic information was collected in a table, among which we calculated the proportion of authors’ and reviewers’ RWI in the interest disclosures in guidelines’ appendixes. Data were mainly extracted from the consideration of the lipid sample profile, lipid-lowering targets (LLTs), statins and other drugs. An alpha level of 0.05 was used to indicate statistical significance. All analyses were performed using SPSS, version 20.0.
Results
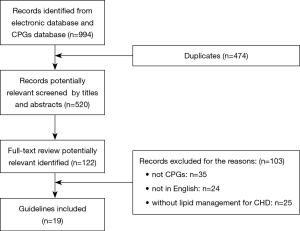
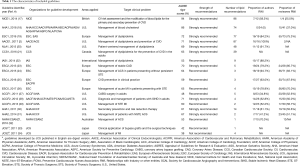
Nineteen guidelines were eventually selected from 994 potentially related articles. Figure 1 was the flow diagram for specific identification. Table 1 listed associated characteristics of included guidelines. Seven of them were dyslipidemia CPGs with recommendations for CHD patients (AACE 2017, NICE 2014, AHA 2018, ESC1 2016, NLA 2015, CCS 2016, IAS 2014) (4,17-22), and twelve of them were CHD guidelines with at least one lipid recommendation (ESC2 2013, ESC3 2015, ESC4 2016, ESC5 2017, ACCF1 2011, ACCF2 2012, ACCF3 2013, AHA1 2011, AHA2 2014, NHFA/CSANZ 2016, JCS1 2011, JCS2 2011) (23-34). Among the 19 included CPGs, IAS was exclusively international, half were from North America, nearly one- third from Europe, only two from Japan and one from Australia and New Zealand. Eligible CPGs were published between 2011–2017.
Full table
Quality assessment using the AGREE II instrument
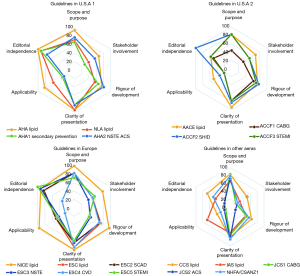
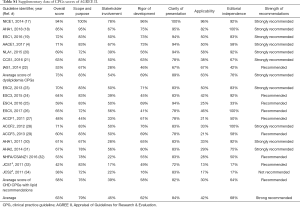
The score of the quality of the included guidelines by AGREE II was illustrated in the 4 radar graphs (Figure 2), and the specific scores could be found in Table S1. Repeatability of the 2 reviewers’ average AGREE II scores was good, with a correlation coefficient of 0.79 in the group.
Full table
Half of the guidelines were “strongly recommended” with scores >60% in more than 4 domains. But the scores of dyslipidemia CPGs were distinctively greater than CHD guidelines in each domain. Only one dyslipidemia and 5 CHD guidelines were “recommended with some modification” on the lipid recommendations. No CPG was described as “not recommended for use in practice”.
Seeing from the score graphs, we could find many universal features. First, guidelines from the same area presented similar score graphs, including CPGs from ESC and CHD guidelines from the U.S.A. Second, dyslipidemia CPGs generally performed better than CHD guidelines, and NICE 2014 (17) scored best. Third, the overall distribution of scores of these domains tended to be consistent, which meant these CPGs existed some common problems under the appraisal system of AGREE II.
For domain 1 “scope and purpose”, most guidelines scored around 80%, and only ACCF1 2011 (27) scored <60%. With the use of AGREE II principles, NICE 2014 (17) even obtained 100% for it clearly defined overall purpose, the clinical questions and target population.
In domain 2, “stakeholder involvement”, only 3 of CPGs (NICE 2014, AHA 2018, AACE 2017) scored >60% (4,17,18). Score lines of other guidelines were more variable and proximal to the center, which resulted in a lower score between 20% and 60%. That may be because none of them presented the views and preferences of the target population. What’s more, most of the CPGs did not include all the relevant experts, especially those from public health, such as statisticians.
For domain 3 “rigor of development”, CPGs from U.S.A got scores between 60% and 80%, scores of European guidelines varied from 20–100% and scores of CPGs in other areas were around 40–60%. Most guidelines described their searching process for evidence and discussed the strengths and limitations of evidence. However, many of CPGs didn’t detail the external review of guidelines. Guidelines scored <60% because they did not present explicit evidence source, key words and the full search strategy.
Domain 4 “clarity of presentation” showed the best consistency in scoring range. And it was the only domain that all guidelines scored >60% among 6 domains. Lipid recommendations in CPGs were identified in forms ranging from colorful charts, distinct flowcharts to summary documents.
In domain 5 “applicability”, seldom did the guidelines score >60% other than NICE 2014, AHA 2018 and IAS 2014 (17,18,22). Several guidelines received scores between 20% and 40%. Rare guidelines introduced facilitators and barriers to the application. Most of them failed to supply advice or tools to the application of recommendations, NICE 2014 (17) was still the exception.
The last domain 6 was “editorial independence”. With the explicit declaration of editorial independence, European guidelines may score best because most of them scored >80% but with ESC4 2016 (25). Most U.S.A guidelines acquired a score >60%. A great part of the eligible CPGs disclosed the RWI of authors (34.8–100%) and reviewers (25–85.7%) in their appendixes. However, most of them merely presented the disclosures of RWI. Only guidelines developed by American organizations defined and disclosed the significant RWI of interests for at least 50,000 dollars, while the specific amount of money and the degree of RWI still remained unknown. It was worth noting that the AHA 2018 writing group members were required to be free of any relevant RWI or be removed. In addition, the digest versions of JCS1 2011 and JCS2 2011 (33,34) scored at the bottom of domain 6 because they did not offer any relative information about funding sources or conflict interests of guidelines development group members.
Comparison of lipid management in guidelines
Dyslipidemia guidelines gave dozens of recommendations on multiple themes, and all CHD patients in dyslipidemia CPGs were classified into the top levels of future atherosclerotic cardiovascular disease (ASCVD) risk. CHD guidelines only presented 4 relevant recommendations on average, but all the included CHD guidelines recommended statin use. While only one third of them made formal recommendations for lipid tests and the target of cholesterol reduction. Less than half of CHD guidelines recommended non-statin drugs.
For the lipid profile, one third of CHD guidelines simply required fasting lipid sample, others didn’t make recommendations. While the latest dyslipidemia management guidelines preferred non-fasting sample, patients with postprandial TGs higher than 4.5 mmol/L (400 mg/dL) required an additional fasting tests in AHA 2018, NLA 2015 and CCS 2016 (18,20,21) (Table 2).

Full table
The lipid lowering targets contained 2 meanings. One was that lipid indicator should be set as the primary target and the other was how much it should be reduced. Eleven CPGs made recommendations on the primary LLT and most of them consistently chose low-density lipoprotein cholesterol (LDL-C). NICE 2014 (17) solely used the non-high-density lipoprotein cholesterol (non-HDL-C) as primary LLT, which was selected as secondary LLT in some CPGs. HDL-C was not selected as the major target and even was not recommended in ESC 2016 and CCS 2016 (19,21).
For the specific targets of cholesterol reduction of CHD patients, most CPGs set targets in 2 aspects. One was the ideal numerical target that was LDL-C <70 mg/dL (1.8 mmol/L). The other was a goal of percentage reduction from fundamental lipid level, which was ≥50% reduction in most guidelines, NICE 2014 (17) uniquely recommended to reduce non-HDL-C >40%. The higher the risk is, the lower the targeted levels of blood lipid need to reach.
Most of CPGs, including CHD guidelines without specific LLTs (ESC2 2013, ACCF2 2012, ACCF3 2013, NHFA/CSANZ 2016, JCS1 2011, JCS2 2011) (23,28,29,32-34), recommended to initiate and maintain statin use, if patients had no contraindication or adverse effects. However, recommendations were rarely given to the specific choice and related dose of statin use. NICE 2014 (17), based on the clinical evidence and economic-effects, was the only one informally recommended atorvastatin as the first choice. In other CPGs, CHD patients were merely recommended to take high intensity of statins, some of whom were recommended the highest tolerable dose to reach the goal.
More than half of the eligible guidelines recommended non-statin therapy for reducing LDL-C or TGs. Drugs including ezetimibe, bile acid sequestrant (BAS), PCSK9 inhibitors aimed at attaining the LDL-C targets, for those CHD patients who were intolerant to statins or unable to reach the goal despite the maximal use of statins. However, with more positive evidence of long-term safety and economic-efficacy ratio, ezetimibe was recommended to use preferentially than PCSK9 inhibitors. The other drugs, containing niacin, fibrate and fish oil were recommended to reduce TGs and prevent HTG patients from acute pancreatitis. With the evidence at low level, drugs included BAS, fibrate, niacin and fish oil were recommended in low grade in most CPGs, NICE 2014 (17) even did not recommend them for secondary prevention. However, AACE 2017 (4) recommended these drugs in the top grade with top-level evidence.
Discussion
In general, we identified 7 dyslipidemia CPGs and 12 CHD guidelines with recommendations on lipid management of CHD patients. Quite a lot of guidelines met the standards of AGREE II recommendation, for most domains scored >30%. Dyslipidemia guidelines commonly provided more detailed lipid recommendations than CHD guidelines. Guidelines reached consensus on treatment that CHD patients should take large or maximally tolerable doses of statins to reduce ASCVD risk; besides, ezetimibe and PCSK9 inhibitors were recommended as second-line therapy. The major inconsistency may exist in the timing for blood tests; the goal chose to mitigate ASCVD risk and aggressive therapy for lowering cholesterols.
Common problems of guidelines’ development were exposed in this systematic assessment. Just like the analysis of another CPGs’ appraisal by the AGREE II instrument (35), previous guidelines almost performed similarly in the 6 domains, greater scores in domain 1 “scope and purpose”, domain 4 “clarity of presentation”, worse in domain 3 “rigor of development”, domain 6 “editorial independence”, and worst in domain 2 “stakeholder involvement”, domain 5 “applicability”. Guidelines of which development referred to or even based on AGREE II instrument won the best scores, such as NICE (17). There were many assessment tools used for evaluating guidelines quality (36), but only AGREE II was validated by an international group of experts. However, more validation should be considered to determine whether the CPGs scores by AGREE II were in line with their true degree of clinical practice and effectiveness of health care.
The sixth domain “editorial independence” with good scores urged us to pay more attention. First, the AGREE scores will not be affected by the high proportion of authors’ and reviewers’ RWI. And then the link between Physicians and the pharmaceutical industry has been found to affect prescribing behavior, even leading to irrational prescription for the company’s drugs (37). Thus the lack of reports on the depth of the relationship between guideline developers and industries was disturbing, and the excessive relationships of RWI should be taken seriously.
As the most updated dyslipidemia CPGs were declined to non-fasting blood sample, and nearly 70% CHD guidelines didn’t show the preference, we speculated that non-fasting tests could be a routine screening in the future, with the complementation of fasting tests. And Dutch laboratories have already drawn postprandial blood sample for lipid tests since 2009. The following reasons may support this conduction. Firstly, blood sample taken after meals would be more accurate, since most of the time we were in non-fasting states (38). More importantly, although the test results of TGs, LDL-C and total cholesterol (TC) were different in both states (39), both fasting and non-fasting tests were likely to achieve the goal of accurately evaluating patients’ CV risk (40-44). What’s more, randomized blood lipid tests did bring convenience in helping clinicians make decisions for ACS patients who needed emergent intervention, and it also would possibly improve CHD patients’ adherence to monitor blood lipid levels (39). In addition, non-fasting tests could be more cost-effective, because after using an adjustable factor for the TG: VLDL-C ratio in the indirect calculation of LDL-C (Friedewald equation: LDL-C = TC − HDL-C − TG/2.2 mmol/L), the difference between both states could be narrowed without additional payment (45,46). Hence, regardless of which specific LLTs was established, non-fasting tests for blood lipid would probably be reliable for CV risk evaluation.
Primary LLT was still LDL-C in guidelines, non-HDL-C and apo B were mainly as secondary targets, because LDL-C reduction was proved to improve clinical outcomes significantly in multiple trials (47-49). But an increasing number of studies may challenge this recommendation, non-HDL-C may become a better target of lipid reduction. On one hand, studies found that non-HDL-C and apo B probably predicted CV risk similarly or even superiorly than LDL-C (50-52). On the other hand, unlike the high cost of apo B, non-HDL-C was possible to predict CV risk accurately in non-fasting conditions and did not require additional fasting tests among HTG patients (17,21,44,46,52).
The specific goals for lipid reduction may follow the principle of “the lower the better”, because either for LDL-C or non-HDL-C, highly intensive statin was currently the most recommended drugs and doses for secondary prevention. Related guidelines made recommendations on LLTs used the words of “at least” and “more than”, because the lower the cholesterol was, the greater the clinical benefits were gained (48,49,53). The LLTs were established because that present treatment limited the attainable LDL-C level around 70 mg/dL (1.8 mmol/L) or a 50% reduction (48,54). For those CHD patients who were unable to meet the goal of LDL-C and non-HDL-C with the maximum tolerated dose of statin, CPGs recommended the use of ezetimibe, which could plus 10–18% reduction (4). But the portion of patients who achieved LLTs was still unsatisfactory (55). Recently, clinical trials of PCSK9 inhibitors obtained unprecedented level of 40 mg/dL (1.0 mmol/L) and 48–71% reduction of LDL-C with the better improvement of clinical outcomes, and no significant short-term adverse reactions were found until now (4,55,56). These studies further proved that lower cholesterol could achieve better clinical benefits. However, the outstanding lipid-lowering effect of PCSK9 inhibitors accompanied with the very high cost, which would prevent drugs from being widely used. What’s more, the long-term clinical benefits and adverse reactions of PCSK9 inhibitors demands further evidence before promoting its use. There are several limitations that should be concerned. First, to make the included CPGs more representative, we removed articles published as an expert consensus, though some of them may meet the standards of CPG. Considering the recognition and application in worldwide, we also excluded CPGs published in other languages instead of English. Second, due to the lack of research funding, we only completed the systematic evaluation of the guidelines through 2 evaluators, rather than the 4 appraisers recommended by the AGREE II tool. However, we had a third reviewer to resolve the discrepancies between the 2 appraisers’ opinions and finally obtained a reliable result of repeatability. Third, AGREE II instrument was merely able to assess the process rigor of guidelines development, but the reliability and the feasibility of recommendations could not be evaluated in this tool. Thus, some recommendations in a guideline with a high assessment score of AGREE II, may not be recognized and followed by clinicians. On the contrary, a guideline with low scores may have a solid recommendation based on strong clinical evidence. For this reason, with other recommendations excluded, we only appraised lipid recommendations for CHD patients in domain 4 “clarity of presentation” and domain 5 “applicability”, which may reduce the possibility of faulty evaluation. And we thought that for a single recommendation, the domain 3 “rigor of development” could be appraised by the applying of classification of recommendation and level of evidence in most of CPGs. Fourth, some guidelines may be underestimated because not all the related information about the CPG development was published. For example, the low score of domain 2 “stakeholder involvement” majorly resulted from the insufficiency of related information about each author’s specialty and the consideration of patients’ preference.
Conclusions
In summary, this study presents the first comprehensive assessment of both the quality of guidelines and current lipid management for CHD patients. With the use of AGREE II, our finding not only show the general quality of most updated guidelines for lipid management is satisfactory and thus we can continue applying these CPGs but also imply that AGREE II could improve guidelines’ development. In addition, guideline developers’ relationship with the industry is worth of more notice. Consensus has been reached on the specific goal of lipid reduction and the intensity of statins use. Further researches are needed to validate the application of non-fasting sample, and non-HDL-C target, as well as the efficacy and safety of ezetimibe and PCSK9 inhibitors.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (81600206), and Natural Science Foundation of Guangdong Province (2016A030310140/20160903).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Collaborators GCOD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151-210. [Crossref] [PubMed]
- Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372:1333-41. [Crossref] [PubMed]
- Roth GA, Nguyen G, Forouzanfar MH, et al. Estimates of global and regional premature cardiovascular mortality in 2025. Circulation 2015;132:1270-82. [Crossref] [PubMed]
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocrine Practice 2017;23:1-87. [Crossref] [PubMed]
- Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937-52. [Crossref] [PubMed]
- Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007;115:450-8. [Crossref] [PubMed]
- Stamler J. WDNJ. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 1986.2823-8. [Crossref] [PubMed]
- Lohr MJF, Lohr. KN. Clinical Practice Guidelines: Directions for a New Program. Washington (DC): National Academies Press (US), 1990.
- Zuiderent-Jerak T, Forland F, Macbeth F. Guidelines should reflect all knowledge, not just clinical trials. BMJ 2012;345:e6702. [Crossref] [PubMed]
- ConsortiumANS. Appraisal of Guidelines for Research & Evaluation II. AGREE II Instrument. Available from: 2009: (Accessed in November 2015).
- Pokharel Y, Akeroyd JM, Virani SS. Cholesterol Guidelines: More Similar Than Different. Prog Cardiovasc Dis 2016;59:190-9. [Crossref] [PubMed]
- Jamé S, Wittenberg E, Potter MB, et al. The New Lipid Guidelines: What Do Primary Care Clinicians Think? Am J Med 2015;128:914.e5-914.e10. [Crossref] [PubMed]
- Virani SS, Pokharel Y, Steinberg L, et al. Provider understanding of the 2013 ACC/AHA cholesterol guideline. J Clin Lipidol 2016;10:497-504.e4. [Crossref] [PubMed]
- Trustworthy IOMU, Guidelines CP. Clinical Practice Guidelines We Can Trust. Washington (DC): National Academies Press (US), 2011.
- He WM, Luo Y, Shui X, et al. Critical appraisal of international guidelines on chronic heart failure: Can China AGREE? Int J Cardiol 2016;203:111-4. [Crossref] [PubMed]
- Ou Y, Goldberg I, Migdal C, et al. A Critical Appraisal and Comparison of the Quality and Recommendations of Glaucoma Clinical Practice Guidelines. Ophthalmology 2011;118:1017-23. [Crossref] [PubMed]
- Anthony W, Rajai A, Lindsay B. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. London: National Institute for Health and Care Excellence (UK), 2014:1-302.
- Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation 2018;2018:CIR0000000000000625.
- Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J 2016;37:2999-3058. [Crossref] [PubMed]
- Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 1—Full Report. J Clin Lipidol 2015;9:129-69. [Crossref] [PubMed]
- Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Canadian Journal of Cardiology 2016;32:1263-82. [Crossref] [PubMed]
- Grundy SM, Arai H, Barter P, et al. An International Atherosclerosis Society Position Paper: Global recommendations for the management of dyslipidemia-Full report. J Clin Lipidol 2014;8:29-60. [Crossref] [PubMed]
- Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J 2013;34:2949-3003. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet J, Mueller C, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Atherosclerosis 2016;252:207-74. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: Executive summary. J Thorac Cardiovasc Surg 2012;143:4-34. [Crossref] [PubMed]
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease. J Am Coll Cardiol 2012;60:e44-164. [Crossref] [PubMed]
- O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol 2013;61:e78-140. [Crossref] [PubMed]
- Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and Other Atherosclerotic Vascular Disease: 2011 Update: A Guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011;124:2458-73. [Crossref] [PubMed]
- Amsterdam EA, Wenger NK, Ralph GB, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non – ST-Elevation Acute Coronary Syndromes. J Am Coll Cardiol 2014;64:e139-228. [Crossref] [PubMed]
- Chew DP, Scott IA, Cullen L, et al. National Heart Foundation of Australia & Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Management of Acute Coronary Syndromes 2016. Heart Lung Circ 2016;25:895-951. [Crossref] [PubMed]
- JCS Joint Working Group. Guidelines for the clinical application of bypass grafts and the surgical techniques (JCS 2011) published in 2012--digest version. Circ J 2013;77:1608-41. [Crossref] [PubMed]
- JCS Joint Working Group. Guidelines for Secondary Prevention of Myocardial Infarction (JCS 2011). Circ J 2013;77:231-48. [Crossref] [PubMed]
- Chakhtoura MT, Nakhoul N, Akl EA, et al. Guidelines on vitamin D replacement in bariatric surgery: Identification and systematic appraisal. Metabolism 2016;65:586-97. [Crossref] [PubMed]
- Siering U, Eikermann M, Hausner E, et al. Appraisal tools for clinical practice guidelines: a systematic review. PLoS One 2013;8:e82915. [Crossref] [PubMed]
- Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians' attitudes and prescribing habits: a systematic review. BMJ Open 2017;7:e016408. [Crossref] [PubMed]
- Nordestgaard BG. A Test in Context: Lipid Profile, Fasting Versus Nonfasting. J Am Coll Cardiol 2017;70:1637-46. [Crossref] [PubMed]
- Nordestgaard BG, Langsted A, Mora S, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points—a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J 2016;37:1944-58. [Crossref] [PubMed]
- Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309-16. [Crossref] [PubMed]
- Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299-308. [Crossref] [PubMed]
- Iso H, Imano H, Yamagishi K, et al. Fasting and non-fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: The Circulatory Risk in Communities Study (CIRCS). Atherosclerosis 2014;237:361-8. [Crossref] [PubMed]
- Langsted A, Freiberg JJ, Tybjaerg-Hansen A, et al. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J Intern Med 2011;270:65-75. [Crossref] [PubMed]
- Doran B, Guo Y, Xu J, et al. Prognostic Value of Fasting Versus Nonfasting Low-Density Lipoprotein Cholesterol Levels on Long-Term Mortality: Insight from the National Health and Nutrition Examination Survey III (NHANES-III). Circulation 2014;130:546-53. [Crossref] [PubMed]
- Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a Novel Method vs the Friedewald Equation for Estimating Low-Density Lipoprotein Cholesterol Levels from the Standard Lipid Profile. JAMA 2013;310:2061. [Crossref] [PubMed]
- Sathiyakumar V, Park J, Golozar A, et al. Fasting Versus Nonfasting and Low-Density Lipoprotein Cholesterol Accuracy. Circulation 2018;137:10-9. [Crossref] [PubMed]
- Cholesterol Treatment Trialists' (CTT) Collaboration, Fulcher J, O'Connell R, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. Lancet 2015;385:1397-1405. [Crossref] [PubMed]
- Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol 2014;64:485-94. [Crossref] [PubMed]
- Silverman MG, Ference BA, Im K, et al. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions. JAMA 2016;316:1289. [Crossref] [PubMed]
- Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL Cholesterol, Non – HDL Cholesterol, and Apolipoprotein B Levels With Risk of Cardiovascular Events Among Patients Treated With Statins:A Meta-analysis. JAMA 2012;307:1302-9. [Crossref] [PubMed]
- Sniderman AD, Williams K, Contois JH, et al. A Meta-Analysis of Low-Density Lipoprotein Cholesterol, Non-High-Density Lipoprotein Cholesterol, and Apolipoprotein B as Markers of Cardiovascular Risk. Circulation: Cardiovascular Quality and Outcomes 2011;4:337-45. [Crossref] [PubMed]
- Thanassoulis G, Williams K, Ye K, et al. Relations of Change in Plasma Levels of LDL-C, Non-HDL-C and apoB With Risk Reduction from Statin Therapy: A Meta-Analysis of Randomized Trials. Journal of the American Heart Association 2014;3:e000759. [Crossref] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010;376:1670-81. [Crossref] [PubMed]
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med 2015;372:2387-97. [Crossref] [PubMed]
- Farnier M, Jones P, Severance R, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: The ODYSSEY OPTIONS II randomized trial. Atherosclerosis 2016;244:138-46. [Crossref] [PubMed]
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017;376:1713-22. [Crossref] [PubMed]