Peroral endoscopic myotomy (POEM) for achalasia
Introduction
Achalasia is a rare motility disorder of the esophagus, resulting from the progressive degeneration of ganglion cells in the myenteric plexus in the lower part of esophagus. Achalasia symptoms are due to failed relaxation of the lower esophageal sphincter (LES) associated with loss of peristalsis and impairment of the deglutitive function. Achalasia incidence is approximately 1.6 cases per 100,000 (1) and is usually present between the ages 25–60; however, new onset of achalasia has been reported in pediatric and elderly populations. Men and women are equally affected with achalasia. The etiology of primary achalasia is unknown, although genetic susceptibility combined with a latent infection of Herpes Simplex Virus (HSV) is suspected to trigger inflammatory changes and a cascading autoimmune process (2). Other diseases can mimic achalasia, causing secondary achalasia such as Chagas disease (3), amyloidosis (4), sarcoidosis (5), neurofibromatosis (6), Fabry disease (7), multiple endocrine neoplasia type 2B (8), and juvenile Sjögren syndrome (9,10).
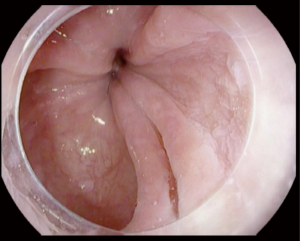
Achalasia is an insidious disease; patients present with symptoms for an average of 4.7 years prior to diagnosis (11). Longstanding achalasia leads to progressive dilatation of the lower esophagus and hypertrophy of the LES (12). Clinical findings may include chest pain (13), weight loss (14), regurgitation and dysphagia (15). Advanced cases are at risk of upper respiratory infections including pneumonia (16), aspiration and lung abscesses (17). Achalasia is diagnosed by a barium swallow and/or manometry (18). Endoscopic evaluation of achalasia is necessary to diagnose conditions that may present as achalasia (pseudoachalasia), for example carcinoma of the gastroesophageal junction (GEJ) (19). Figure 1 illustrate tight GEJ in patient with achalasia.
Treatment of achalasia is aimed at lowering the resting pressure of the LES (20). This can be achieved by a pharmacologic reduction of the muscular resting pressure, botulinum toxin (BT) injection, use of oral nitrates, physical disruption of muscular bundles of LES by laparoscopic myotomy, pneumatic dilation or POEM (18). There is no known treatment to reverse the degenerative process of ganglion cells nor restore normal esophageal function; hence, repeated intervention and long-term follow up is necessary for these patients (21). BT injection is advantageous as a minimally invasive intervention with immediate response in 70–90% of the patients (22-24), but many patients relapse in a few months (25-27). It carries a slight increased risk of perforation (28), ulcers (29) and chest pain (30). Pneumatic dilatation (PD) is forceful mechanical disruption applied to the LES. It is completed by passing a pneumatic balloon with increasing caliber to stretch the circumferential muscle fibers (31). PD should be performed by an experienced endoscopist. Patients undergoing PD should be good surgical candidates due to the risk of perforation requiring surgical intervention (32). PD is the most cost-effective intervention (33) and initial response is high; however, efficacy wanes over time (34). Immediate complications include perforation in 2% and heartburn in 15–35% (34,35).
Heller myotomy was the primary therapy for achalasia after PD failure, it is usually performed laparoscopically (35-37). Due to the disruptive nature of the intervention, it frequently causes reflux esophagitis and is frequently combined with anti-reflux intervention (37). Heller myotomy is the least cost-effective intervention for achalasia (33), with initial symptom relief in 90% of patients (35); however, it has a long recovery period, in addition to the risk of perforation, bleeding and infection (38).
POEM was first introduced by Ortega JA in 1980. In his initial report, seventeen patients with achalasia were treated by endoscopic myotomy limited to esophageal rosette. In this cohort improvement of symptoms and manometry follow-up was comparable to Heller myotomy (39). The current form of POEM was developed by Inoue in 2008 (40). He utilized a submucosal tunnel to reach the inner circular muscle bundle of the LES to perform the myotomy (41). Following the initial publication, Inoue et al. presented their experience in performing POEM on 43 patients for the treatment of achalasia. The authors achieved a comparable outcome to Heller’s myotomy (42). POEM is emerging as the treatment of choice for achalasia and is even utilized for prior failed achalasia treatment including laparoscopic surgical myotomy (43). POEM is also applied to treat other motility disorders including spastic esophageal disorders (SED), such as diffuse esophageal spasm, jackhammer esophagus, or type 3 achalasia (44). In a meta-analysis of nine studies with 210 patients, Chandan et al. found that POEM was safe and effective in treatment of the SED in over 89% of cases.
Pre-operative evaluation
In the pre-operative evaluation of achalasia, barium swallow, esophageal manometry and EGD should be performed to confirm diagnosis and exclude other conditions (e.g., cancer). High resolution manometry allows tailored treatment based on the type of achalasia.
Patient should be on a clear liquid diet for 2 days before the procedure and NPO the night of the procedure. In some centers, EGD is performed before general anesthesia to remove food remnants and assess for candida esophagitis (45); another approach is to place a nasogastric tube for suction 1 to 2 days prior to the procedure. Oral antifungal treatment can be administered one week prior to the procedure if candidiasis is suspected. A broad-spectrum antibiotic is usually given intravenously the day of the procedure. Anticoagulants and anti-platelet medication should be withheld prior to the intervention.
Technique
A forward viewing scope with a transparent distal cap and triangle or rounded tip knife is used to dissect the submucosal layer and cut the inner circular muscle bundles. A coagulating grasper may be used for hemostasis.
Step 1: mucosal incision
Using a mixture of epinephrine and indigo carmine or methylene blue, a submucosal bleb is raised and a mucosal incision is performed. The incision is done longitudinally with careful dissection of the submucosal fibers around the linear incision to allow the gastroscope to be introduced into the submucosal space. Longitudinal incision facilitates closure with endoscopic clips; in some occasions, a horizontal incision is done to allow suturing of the incision site.
In most cases, submucosal tunnel and myotomy are performed at an anterior position, 2 o’clock, in other cases, a posterior position at 5 o’clock is preferred. A randomized trial comparing the two approaches in 32 patients, found no difference in efficacy nor complications (46). In another study of 448 patients, posterior POEM was associated with fewer adverse events, lower risk of mucosectomy and a shorter incision closure time (47).
In failed surgical attempts or after previous anterior POEM failure, posterior POEM is performed to avoid previous surgical site scarring (48).
Step 2: creating a submucosal tunnel
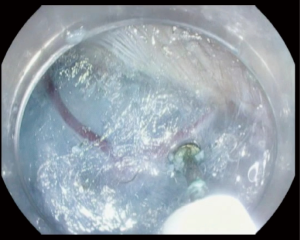
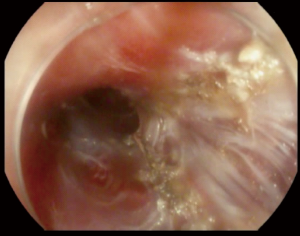
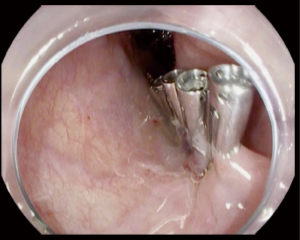
After creation of the entry site, the endoscope is advanced within the submucosa while preserving the integrity of the mucosa. It is very important to preserve the mucosa since it will be the only remaining barrier between the mediastinum and esophageal lumen after myotomy (Figure 2). Typically, dry cut current, forced coagulation current or spry coagulation are used to dissect the submucosa after repeated injection of saline with methylene blue. Larger blood vessels in the submucosa are usually coagulated using hemostatic forceps (Figure 3).
The GEJ is identified by multiple methods including visualization of the longitudinal muscle bundles at the GEJ, narrowing of the submucosal space and resistance of advancing the endoscope thought GEJ, followed by expansion of the space in the gastric cardia. The appearance of spiral or comma shaped small blood vessels in the submucosa is another indicator. Once the gastro esophageal junction (GEJ) is identified, the endoscope should be advanced 2 to 3 cm beyond it. On some occasions, prior to tunnel creation, the lower most part of the tunnel is injected 2 cm below the GEJ with indocyanine green to mark the extent of tunnel (40,49).
Step 3: myotomy
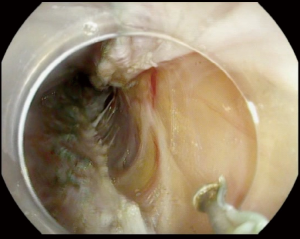
Selective myotomy of the inner circular muscle bundles is performed starting 6 cm above the GEJ and extends 2–3 cm below the GEJ (Figure 4). The selective myotomy of the inner circular layer while preserving the longitudinal outer layer may be difficult to achieve and is time consuming. The longitudinal outer muscle layer is very thin and fragile making selective myotomy difficult. May endoscopists advocate for full thickness myotomy (Figure 5). In a study of 103 patients comparing the selective myotomy of inner circular bundles versus full-thickness myotomy, it was found that short-term relief and clinical outcome of both methods were comparable (50). Full-thickness myotomy significantly reduced the procedure time without increase in adverse events or reflux (50). However, 24-hour pH study in the same trial showed that abnormal esophageal acid exposure was higher in the full-thickness myotomy group when compared to the selective myotomy group; although not statistically significant (P>0.05) (50).
Step 4: closure of mucosal incision
After successful completion of the myotomy, careful inspection of the submucosal tunnel should be performed. The endoscopist should ensure that any active bleeding is controlled prior to closure. The esophageal mucosa is then inspected and any incidental tear, mucosectomy, should be closed. Closure of the initial mucosal incision can be performed with endoscopic clips or endoscopic suturing devices (Figure 6).
Post-operative care
A gastrografin swallow study with fluoroscopy should be obtained to confirm the absence of any leakage. A soft diet can be started on day 2 post operatively and continue for 10–14 days before starting a regular diet. Intravenous antibiotics should be stopped on day 3 and switched to oral antibiotics for a total of 7 days. Proton pump inhibitors should be prescribed for a minimum of 14 days.
Follow-up
A 3–6-month post-procedure follow-up should include EGD, manometry and a pH study to evaluate the patient’s outcome and assess any complications.
Contraindications
Contraindications to the procedure include severe esophagitis, significant coagulation disorder, advanced liver cirrhosis and submucosal fibrosis from prior radiation. Large esophageal diverticulum is considered a relative contraindication depending on the location and the extent of the diverticulum (Figure 7 illustrate esophageal diverticulum in a patient with achalasia).
Outcomes of POEM for achalasia
POEM aims primarily to treat achalasia. The intervention is successful and efficacious for the management of symptoms with a success rate of 82–100% (45,51). Outcomes of the intervention to treat achalasia is assessed subjectively and objectively by multiple methods. The most used tool to assess outcomes is the Eckardt score, which is a subjective assessment of pre and post-intervention symptoms (see Table 1). Clinical success is defined as a post-intervention Eckardt score of 3 or lower or a reduction of LES by 50% or more (45). Other measures to assess clinical improvement such as quality of life (53), or a barium swallow (54) also show similar favorable outcomes after POEM.
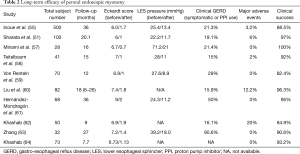
Intermediate to long-term outcome using the Eckardt score was studied in Japan and the USA. Both studies observed 90% clinical success at 24-month after the intervention (55,56) (Table 2).
Full table
When compared to Laparoscopic Heller Myotomy (LHM) (Table 3), POEM was shown to have similar safety and efficacy (65). In one meta-analysis of 486 patients who received POEM, it was found that they had a similar reduction of Eckardt score compared to LHM. In another meta-analysis of over 7,000 patients including over 70 cohort studies, POEM was more effective than LHM, improving dysphagia (66) at 12-, 24-, 30- (56) and 60-month (61), although POEM was found to have higher incidents of new gastro-esophageal reflux disease (GERD). New suggested approaches include combining POEM with anti-reflux measures (67); in this series, patients who underwent POEM and transoral incisional fundoplication (TIF) found to have improved symptoms of esophagitis and lower need for long-term proton pump inhibitor (PPI) use. POEM improved all dimensions of health-related quality of life in one study of 143 patients. Perbtani et al., showed significant improvement of SF-36 survey scores in a long-term follow-up study (16.4 months with a range of 12 to 40 months). The survey was obtained before and after POEM and it showed a strong association of Eckardt score improvements in all health-related quality of life (68).

Full table
POEM for recurrent achalasia
POEM is also a feasible and safe option for treatment of recurrent achalasia. Patients who failed in previous endoscopic or laparoscopic attempts underwent successful POEM and had a favorable clinical response with an Eckardt score of 3 or less (69-71). Sharata et al. reported the outcomes of POEM in 40 achalasia patients who failed a prior BT or pneumatic dilation. POEM resulted in a favorable outcome with an Eckardt score 3 or less. Of note, previous treatment with PD and or BT was not associated with increased intra- or post-operative adverse events (69). Another study of twenty-one patients found a similar outcome after POEM in patients who failed prior repeated BT and PD therapy (70). Orenstein et al., reported the outcome of POEM in a forty-one patients with achalasia who previously failed endoscopic treatment or surgery (LHM). In Both group, there was no difference in POEM outcome or adverse events (71). however submucosal fibrosis from repeated BT and PD rendered dissection more difficult (49). In a retrospective multi-center study which compared the POEM outcomes of ninety patients with prior LHM compared to ninety patients without previous intervention, the adverse event rate was similar; however, clinical success was lower in the group who previously received LHM (94% vs. 81%) (72). Another study that looked at repeated POEM in patients with previously failed POEM found average Eckardt score improved from 4.3 to 1.64 (73).
Adverse events
In experienced hands, POEM is a safe procedure with low post-operative adverse events (74). Adverse events are usually managed medically or endoscopically. In a multi-center international study that included 1,826 patients, adverse events ranging from mild to severe occurred in 137 patients with nine patients experiencing a severe adverse event (75).
Mucosal tear
Mucosal tear during POEM requires closure because it represent a full-thickness esophageal perforation. Mucosal tear has a highest risk of occurrence at level of LES due to narrowing of submucosal space. Mucosal tear tends to expand quickly if not addressed immediately; it is usually closed with endoclips, although glue (76) and over-the-scope clips are also used (77). Mucosal tear can lead to mediastinitis if not treated (45).
Bleeding
Bleeding during the submucosal dissection is expected and addressed with multiple methods including pressure with gastroscope tip, electrocautery knife or hemostatic forceps. In one study, delayed bleeding occurred in 0.7% (78). Hematemesis after POEM is an emergency; the patient should undergo immediate endoscopy to assess the surgical site.
GERD
GERD is the most common adverse event post-POEM, with a prevalence rate of 20–57% (45). As mentioned above, combing POEM with TIF improved GERD and esophagitis. Other methods to decrease GERD post-POEM include preserving the outer longitudinal muscle bundles and the sling fibers. In comparison to full thickness myotomy, selective myotomy was associated with a lower esophageal acid exposure based on 24 hours manometry study in some published trials (50). Recently it was suggested to limit the extension of the myotomy to only one centimeter beyond GEJ (instead of 3 cm); however, this has not yet been fully studied.
Pneumoperitoneum
Small pneumoperitoneum occurs in 50% of cases and subcutaneous emphysema occurs in 15% (45). Both events resolve spontaneously. Tension pneumoperitoneum is rare and can be assessed clinically by an abdominal exam, it can be addressed with prompt decompression using a large bore needle.
Pneumothorax
Pneumothorax is a rare event, usually left to resolve spontaneously, unless respiratory compromise occurs.
Tailoring POEM to the type of achalasia
Based on esophageal pressurization by high resolution manometry, Chicago Classification (CC v3.0) identify 3 subtypes of achalasia (79):
- type I classic: 100% failed peristalsis, swallowing results in no significant change in esophageal pressurization;
- type II: 100% failed peristalsis, swallowing results in pan esophageal pressurization with ≥20% of swallows;
- type III: swallowing results in abnormal spasms, no normal peristalsis, premature (spastic) contractions with distal contractile integral >450 mmHg-s-cm in ≥20% of swallows.
Standard POEM technique is tailored for type I and type II. Performing the myotomy according to the length of the spastic segment is required in type III. In type III achalasia which is characterized by rapidly propagating pressure due to spastic contractions, POEM was superior to LHM due to longer myotomy 16 vs. 8 cm, shorter procedure time (80) and significant better clinical outcome (98.0% vs. 80.8%).
POEM training
POEM is a complex procedure, demanding skilled hands to avoid serious complications. Endoscopists should be able to recognize structures beyond mucosa, including vasculature nerves and the anatomy of the mediastinum. It is currently performed in highly specialized centers by experienced endoscopists or surgeons. Initially the endoscopist should observe the procedure performed by experienced operators, familiarize themselves with all equipment needed for the intervention including the tools to control possible adverse events. After that endoscopist should perform POEM on animal models to develop the needed skills. The most commonly used animal model is swine esophagus. Swine esophagus is long which allows submucosal dissection and myotomy, replicating the experience in human. Swine model disadvantages include a soft and avascular submucosal space allowing easier dissection in comparison to human, however, the swine muscle layer is thinner with a higher risk of perforation. Training on animal models should go through two phases, non-survival animal model followed by survival models using the same equipment and principles described for humans. After evaluation of a successful training in the above structured program, endoscopists who can meet the competency requirements can advance to perform POEM in tertiary centers (48).
Acknowledgments
None.
Footnote
Conflicts of Interest: MO Othman MD, is a consultant for Olympus, Boston Scientific, Abbvie and Lumendi. Y Ahmed has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Sadowski DC, Ackah F, Jiang B, et al. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil 2010;22:e256-61. [Crossref] [PubMed]
- Facco M, Brun P, Baesso I, et al. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol 2008;103:1598-609. [Crossref] [PubMed]
- de Oliveira RB, Rezende Filho J, Dantas RO, et al. The spectrum of esophageal motor disorders in Chagas' disease. Am J Gastroenterol 1995;90:1119-24. [PubMed]
- Costigan DJ, Clouse RE. Achalasia-like esophagus from amyloidosis. Successful treatment with pneumatic bag dilatation. Dig Dis Sci 1983;28:763-5. [Crossref] [PubMed]
- Dufresne CR, Jeyasingham K, Baker RR. Achalasia of the cardia associated with pulmonary sarcoidosis. Surgery 1983;94:32-5. [PubMed]
- Foster PN, Stewart M, Lowe JS, et al. Achalasia like disorder of the oesophagus in von Recklinghausen's neurofibromatosis. Gut 1987;28:1522-6. [Crossref] [PubMed]
- Roberts DH, Gilmore IT. Achalasia in Anderson-Fabry's disease. J R Soc Med 1984;77:430-1. [PubMed]
- Cuthbert JA, Gallagher ND, Turtle JR. Colonic and oesophageal disturbance in a patient with multiple endocrine neoplasia, type 2b. Aust N Z J Med 1978;8:518-20. [Crossref] [PubMed]
- Simila S, Kokkonen J, Kaski M. Achalasia sicca--juvenile Sjogren's syndrome with achalasia and gastric hyposecretion. Eur J Pediatr 1978;129:175-81. [Crossref] [PubMed]
- Schuffler MD. Chronic intestinal pseudo-obstruction syndromes. Med Clin North Am 1981;65:1331-58. [Crossref] [PubMed]
- Eckardt VF, Kohne U, Junginger T, et al. Risk factors for diagnostic delay in achalasia. Dig Dis Sci 1997;42:580-5. [Crossref] [PubMed]
- Seeman H, Traube M. Hiccups and achalasia. Ann Intern Med 1991;115:711-2. [Crossref] [PubMed]
- Eckardt VF, Stauf B, Bernhard G. Chest pain in achalasia: patient characteristics and clinical course. Gastroenterology 1999;116:1300-4. [Crossref] [PubMed]
- Howard PJ, Maher L, Pryde A, et al. Five year prospective study of the incidence, clinical features, and diagnosis of achalasia in Edinburgh. Gut 1992;33:1011-5. [Crossref] [PubMed]
- Fisichella PM, Raz D, Palazzo F, et al. Clinical, radiological, and manometric profile in 145 patients with untreated achalasia. World J Surg 2008;32:1974-9. [Crossref] [PubMed]
- Mashkov AE, Pykchteev DA, Sigachev AV, et al. Obstructive bronchitis and recurrent pneumonia in esophageal achalasia in a child: A CARE compliant case report. Medicine (Baltimore) 2018;97:e11016. [Crossref] [PubMed]
- Bansal R, Sikder T, Aron J, et al. Lung Abscess: Atypical Presentation of Achalasia. Am J Med Sci 2016;351:407. [Crossref] [PubMed]
- Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol 2013;108:1238-49. [Crossref] [PubMed]
- Carter M, Deckmann RC, Smith RC, et al. Differentiation of achalasia from pseudoachalasia by computed tomography. Am J Gastroenterol 1997;92:624-8. [PubMed]
- Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet 2014;383:83-93. [Crossref] [PubMed]
- Kahrilas PJ. Treating achalasia; more than just flipping a coin. Gut 2016;65:726-7. [Crossref] [PubMed]
- Pasricha PJ, Ravich WJ, Hendrix TR, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med 1995;332:774-8. [Crossref] [PubMed]
- Cuilliere C, Ducrotte P, Zerbib F, et al. Achalasia: outcome of patients treated with intrasphincteric injection of botulinum toxin. Gut 1997;41:87-92. [Crossref] [PubMed]
- Rollan A, Gonzalez R, Carvajal S, et al. Endoscopic intrasphincteric injection of botulinum toxin for the treatment of achalasia. J Clin Gastroenterol 1995;20:189-91. [Crossref] [PubMed]
- Allescher HD, Storr M, Seige M, et al. Treatment of achalasia: botulinum toxin injection vs. pneumatic balloon dilation. A prospective study with long-term follow-Up. Endoscopy 2001;33:1007-17. [Crossref] [PubMed]
- Bassotti G, D'Onofrio V, Battaglia E, et al. Treatment with botulinum toxin of octo-nonagerians with oesophageal achalasia: a two-year follow-up study. Aliment Pharmacol Ther 2006;23:1615-9. [Crossref] [PubMed]
- Annese V, Bassotti G, Coccia G, et al. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. GISMAD Achalasia Study Group. Gut 2000;46:597-600. [Crossref] [PubMed]
- Weusten BL, Samsom M, Smout AJ. Pneumothorax complicating botulinum toxin injection in the body of a dilated oesophagus in achalasia. Eur J Gastroenterol Hepatol 2003;15:561-4. [Crossref] [PubMed]
- Fitzgerald JF, Troncone R, Sukerek H, et al. Clinical quiz. Sinus tract between esophagus and fundus. J Pediatr Gastroenterol Nutr 2002;35:38, 98.
- Eaker EY, Gordon JM, Vogel SB. Untoward effects of esophageal botulinum toxin injection in the treatment of achalasia. Dig Dis Sci 1997;42:724-7. [Crossref] [PubMed]
- Borhan-Manesh F, Kaviani MJ, Taghavi AR. The efficacy of balloon dilation in achalasia is the result of stretching of the lower esophageal sphincter, not muscular disruption. Dis Esophagus 2016;29:262-6. [Crossref] [PubMed]
- Ghoshal UC, Karyampudi A, Verma A, et al. Perforation following pneumatic dilation of achalasia cardia in a university hospital in northern India: A two-decade experience. Indian J Gastroenterol 2018;37:347-52. [Crossref] [PubMed]
- Kostic S, Johnsson E, Kjellin A, et al. Health economic evaluation of therapeutic strategies in patients with idiopathic achalasia: results of a randomized trial comparing pneumatic dilatation with laparoscopic cardiomyotomy. Surg Endosc 2007;21:1184-9. [Crossref] [PubMed]
- Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic Heller's myotomy for idiopathic achalasia. N Engl J Med 2011;364:1807-16. [Crossref] [PubMed]
- Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 2009;249:45-57. [Crossref] [PubMed]
- Hunter JG, Trus TL, Branum GD, et al. Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg 1997;225:655-64; discussion 664-5. [Crossref] [PubMed]
- Vogt D, Curet M, Pitcher D, et al. Successful treatment of esophageal achalasia with laparoscopic Heller myotomy and Toupet fundoplication. Am J Surg 1997;174:709-14. [Crossref] [PubMed]
- Lynch KL, Pandolfino JE, Howden CW, et al. Major complications of pneumatic dilation and Heller myotomy for achalasia: single-center experience and systematic review of the literature. Am J Gastroenterol 2012;107:1817-25. [Crossref] [PubMed]
- Ortega JA, Madureri V, Perez L. Endoscopic myotomy in the treatment of achalasia. Gastrointest Endosc 1980;26:8-10. [Crossref] [PubMed]
- Minami H, Inoue H, Haji A, et al. Per-oral endoscopic myotomy: emerging indications and evolving techniques. Dig Endosc 2015;27:175-81. [Crossref] [PubMed]
- Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. [Crossref] [PubMed]
- Inoue H, Kudo SE. Per-oral endoscopic myotomy (POEM) for 43 consecutive cases of esophageal achalasia. Nihon Rinsho 2010;68:1749-52. [PubMed]
- Youn YH, Minami H, Chiu PW, et al. Peroral Endoscopic Myotomy for Treating Achalasia and Esophageal Motility Disorders. J Neurogastroenterol Motil 2016;22:14-24. [Crossref] [PubMed]
- Chandan S, Mohan BP, Chandan OC, et al. Clinical efficacy of per-oral endoscopic myotomy (POEM) for spastic esophageal disorders: a systematic review and meta-analysis. Surg Endosc 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Stavropoulos SN, Desilets DJ, Fuchs KH, et al. Per-oral endoscopic myotomy white paper summary. Gastrointestinal Endoscopy 2014;80:1-15. [Crossref] [PubMed]
- Tan Y, Lv L, Wang X, et al. Efficacy of anterior versus posterior per-oral endoscopic myotomy for treating achalasia: a randomized, prospective study. Gastrointest Endosc 2018;88:46-54. [Crossref] [PubMed]
- Rodriguez de Santiago E, Mohammed N, Manolakis A, et al. Anterior versus posterior myotomy during poem for the treatment of achalasia: systematic review and meta-analysis of randomized clinical trials. J Gastrointestin Liver Dis 2019;28:107-15. [PubMed]
- Eleftheriadis N, Inoue H, Ikeda H, et al. Training in peroral endoscopic myotomy (POEM) for esophageal achalasia. Therapeutics and clinical risk management 2012;8:329-42. [Crossref] [PubMed]
- Stavropoulos SN, Modayil RJ, Friedel D, et al. The International Per Oral Endoscopic Myotomy Survey (IPOEMS): a snapshot of the global POEM experience. Surg Endosc 2013;27:3322-38. [Crossref] [PubMed]
- Li QL, Chen WF, Zhou PH, et al. Peroral endoscopic myotomy for the treatment of achalasia: a clinical comparative study of endoscopic full-thickness and circular muscle myotomy. J Am Coll Surg 2013;217:442-51. [Crossref] [PubMed]
- Sharata AM, Dunst CM, Pescarus R, et al. Peroral endoscopic myotomy (POEM) for esophageal primary motility disorders: analysis of 100 consecutive patients. J Gastrointest Surg 2015;19:161-70; discussion 170. [Crossref] [PubMed]
- Chandrasekhara V, Desilets D, Falk GW, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on peroral endoscopic myotomy. Gastrointest Endosc 2015;81:1087-100.e1. [Crossref] [PubMed]
- Vigneswaran Y, Tanaka R, Gitelis M, et al. Quality of life assessment after peroral endoscopic myotomy. Surg Endosc 2015;29:1198-202. [Crossref] [PubMed]
- Verlaan T, Rohof WO, Bredenoord AJ, et al. Effect of peroral endoscopic myotomy on esophagogastric junction physiology in patients with achalasia. Gastrointest Endosc 2013;78:39-44. [Crossref] [PubMed]
- Inoue H, Sato H, Ikeda H, et al. Per-Oral Endoscopic Myotomy: A Series of 500 Patients. J Am Coll Surg 2015;221:256-64. [Crossref] [PubMed]
- Hungness ES, Sternbach JM, Teitelbaum EN, et al. Per-oral Endoscopic Myotomy (POEM) After the Learning Curve: Durable Long-term Results With a Low Complication Rate. Ann Surg 2016;264:508-17. [Crossref] [PubMed]
- Minami H, Isomoto H, Yamaguchi N, et al. Peroral endoscopic myotomy for esophageal achalasia: Clinical impact of 28 cases. Dig Endosc 2014;26:43-51. [Crossref] [PubMed]
- Teitelbaum EN, Soper NJ, Santos BF, et al. Symptomatic and physiologic outcomes one year after peroral esophageal myotomy (POEM) for treatment of achalasia. Surg Endosc 2014;28:3359-65. [Crossref] [PubMed]
- Von Renteln D, Fuchs KH, Fockens P, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology 2013;145:309-11.e1-3.
- Liu W, Zeng XH, Yuan XL, et al. Open peroral endoscopic myotomy for the treatment of achalasia: a case series of 82 cases. Dis Esophagus 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Hernández-Mondragón OV, Solorzano-Pineda OM, Gonzalez-Martinez M, et al. Gastroesophageal reflux disease after peroral endoscopic myotomy: Short-term, medium-term, and long-term results. Rev Gastroenterol Mex 2019. [Epub ahead of print]. [PubMed]
- Khashab MA, Familiari P, Draganov PV, et al. Peroral endoscopic myotomy is effective and safe in non-achalasia esophageal motility disorders: an international multicenter study. Endosc Int Open 2018;6:E1031-e6. [Crossref] [PubMed]
- Zhang W, Linghu EQ. Peroral Endoscopic Myotomy for Type III Achalasia of Chicago Classification: Outcomes with a Minimum Follow-Up of 24 Months. J Gastrointest Surg 2017;21:785-91. [Crossref] [PubMed]
- Khashab MA, Messallam AA, Onimaru M, et al. International multicenter experience with peroral endoscopic myotomy for the treatment of spastic esophageal disorders refractory to medical therapy (with video). Gastrointest Endosc 2015;81:1170-7. [Crossref] [PubMed]
- Marano L, Pallabazzer G, Solito B, et al. Surgery or Peroral Esophageal Myotomy for Achalasia: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2016;95:e3001. [Crossref] [PubMed]
- Schlottmann F, Luckett DJ, Fine J, et al. Laparoscopic Heller Myotomy Versus Peroral Endoscopic Myotomy (POEM) for Achalasia: A Systematic Review and Meta-analysis. Ann Surg 2018;267:451-60. [Crossref] [PubMed]
- Tyberg A, Choi A, Gaidhane M, et al. Transoral incisional fundoplication for reflux after peroral endoscopic myotomy: a crucial addition to our arsenal. Endosc Int Open 2018;6:E549-52. [Crossref] [PubMed]
- Perbtani YB, Mramba LK, Yang D, et al. Life after per-oral endoscopic myotomy: long-term outcomes of quality of life and their association with Eckardt scores. Gastrointest Endosc 2018;87:1415-20.e1. [Crossref] [PubMed]
- Sharata A, Kurian AA, Dunst CM, et al. Peroral endoscopic myotomy (POEM) is safe and effective in the setting of prior endoscopic intervention. J Gastrointest Surg 2013;17:1188-92. [Crossref] [PubMed]
- Ling T, Guo H, Zou X. Effect of peroral endoscopic myotomy in achalasia patients with failure of prior pneumatic dilation: a prospective case-control study. J Gastroenterol Hepatol 2014;29:1609-13. [Crossref] [PubMed]
- Orenstein SB, Raigani S, Wu YV, et al. Peroral endoscopic myotomy (POEM) leads to similar results in patients with and without prior endoscopic or surgical therapy. Surg Endosc 2015;29:1064-70. [Crossref] [PubMed]
- Ngamruengphong S, Inoue H, Ujiki MB, et al. Efficacy and Safety of Peroral Endoscopic Myotomy for Treatment of Achalasia After Failed Heller Myotomy. Clin Gastroenterol Hepatol 2017;15:1531-7.e3. [Crossref] [PubMed]
- Tyberg A, Seewald S, Sharaiha RZ, et al. A multicenter international registry of redo per-oral endoscopic myotomy (POEM) after failed POEM. Gastrointest Endosc 2017;85:1208-11. [Crossref] [PubMed]
- Werner YB, von Renteln D, Noder T, et al. Early adverse events of per-oral endoscopic myotomy. Gastrointest Endosc 2017;85:708-18.e2. [Crossref] [PubMed]
- Haito-Chavez Y, Inoue H, Beard KW, et al. Comprehensive Analysis of Adverse Events Associated With Per Oral Endoscopic Myotomy in 1826 Patients: An International Multicenter Study. Am J Gastroenterol 2017;112:1267-76. [Crossref] [PubMed]
- Li H, Linghu E, Wang X. Fibrin sealant for closure of mucosal penetration at the cardia during peroral endoscopic myotomy (POEM). Endoscopy 2012;44 Suppl 2 UCTN:E215-6.
- Kumbhari V, Azola A, Saxena P, et al. Closure methods in submucosal endoscopy. Gastrointest Endosc 2014;80:894-5. [Crossref] [PubMed]
- Li QL, Zhou PH, Yao LQ, et al. Early diagnosis and management of delayed bleeding in the submucosal tunnel after peroral endoscopic myotomy for achalasia (with video). Gastrointest Endosc 2013;78:370-4. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Kumbhari V, Tieu AH, Onimaru M, et al. Peroral endoscopic myotomy (POEM) vs laparoscopic Heller myotomy (LHM) for the treatment of Type III achalasia in 75 patients: a multicenter comparative study. Endosc Int Open 2015;3:E195-201. [Crossref] [PubMed]