
Surgical approach for thymic epithelial tumor
Resection continues to be the mainstay treatment for thymic epithelial tumors (1,2), with complete removal of the tumor and involved organs the ultimate aim. As noted in the study by Carretta, the choice of surgical approach has a major role in determining treatment success, thus the optimal chosen method should provide adequate surgical view in order to achieve complete tumor resection.
Although a video-assisted thoracoscopic surgery (VATS) thymectomy for thymoma has been introduced, the oncological outcome of that procedure remains unclear. We previously reported that a VATS thymectomy is feasible for early-stage thymoma cases, though the indications must be carefully considered in patients with thymoma larger than 5 cm or cystic thymoma (3). Additionally, we also perform a thymic carcinoma resection using a median sternotomy because it provides for complete dissection of the anterior mediastinal lymph nodes (4,5).
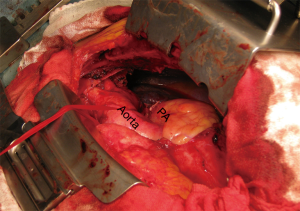
While a median sternotomy is considered to be the gold standard for access to the mediastinum, that approach does not provide an adequate surgical view for accessing the upper and lower chest cavity (6). For bulky tumors or pulmonary hilar involvement, a median sternotomy may not allow an adequate surgical view, thus we consider VATS in addition to a median sternotomy to be useful to examine the mediastinum behind the tumor or pulmonary hilum. Furthermore, the surgical view provided by VATS is especially helpful to detect, as well as dissect and preserve the phrenic nerve adjacent to the mediastinal tumor by manipulation with a median sternotomy (Figure 1). In such cases, we use an internal mammary artery (IMA) retractor system to obtain a wider surgical view of the thoracic cavity, which allows the cardiac surgeon to perform gentle controlled lifting of the chest wall for harvesting the IMA (Figure 2).

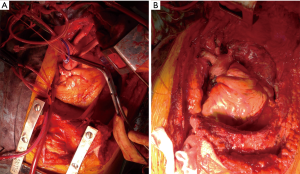
Nevertheless, these approaches have significant limitations in cases with an advanced thymic tumor involving the great vessels and pulmonary hilum. We recently reported that a hemi-clamshell (HCS) approach provided excellent exposure of tumor-infiltrated organs in patients with thymic malignancy (7). Additionally, use of the HCS approach, first reported by Masaoka et al., a member of our department at that time, has been shown for surgical treatment of cervico-thoracic and mediastinal lesions (8), and we often employ it for resection of thoracic malignancy, such as lung cancer cases (9,10). For successful lung resection in patients with a thymic tumor, the HCS approach is useful, as it provides a direct view from multiple directions of access to the tumor and hilum, as well as the interlobar surface, and is also beneficial for resection of disseminated lesions and diaphragmatic plication. When requiring an extended thymectomy for myasthenia gravis or extended tumor invasion to bilateral thymus tissue, a tumor contralateral lesion should be resected via a sternotomy, followed by the HCS approach, because the surgical view for a contralateral lesion is often better with a full sternotomy as compared to an HCS approach with a partial sternotomy. Based on our experience, we consider that for treating patients with a thymic tumor on the left side of the thymus who require a cardiopulmonary bypass for the resection of the tumor and involved organs, such as the aorta, pulmonary artery, or left atrial, a full sternotomy should be performed first to secure the right side of the heart, including the superior vena cava, inferior vena cava, and right atrial, so as to obtain blood access for a cardiopulmonary bypass before employing a left thoracotomy with the HCS approach (Figure 3).
We consider that a pleuropneumonectomy is a feasible approach for cases with a large thymoma with invasion to the hilum of the lung and pleural dissemination (11). Kondo et al. reported that thymoma shows moderate response to chemotherapy or radiotherapy, which appears to be increasing the rate of complete resection procedures as well as survival of patients with an advanced thymoma (12). In addition, surgical debulking is acceptable for thymoma, because of the potential for a favorable outcome (13). We agree with that concept, and prefer conservative treatment with resection of visible disseminated nodules by use of a partial pleurectomy for patients with a stage IVa thymoma via a median sternotomy with or without VATS, as well as an HCS approach. Although an HCS method facilitates exposure of the pleural cavity, that is not adequate for a pleuropneumonectomy, and both a median sternotomy and posterolateral thoracotomy are required for a pleuropneumonectomy with patient repositioning.
In conclusion, we fully agree with Carretta’s comments, and consider that different surgical approaches according to tumor stage and size, involved organs, and thymic tumor histology, as well as surgeon preference and expertise should be proposed.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Kondo K. Optimal therapy for thymoma. J Med Invest 2008;55:17-28. [Crossref] [PubMed]
- Kimura T, Inoue M, Kadota Y, et al. The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2013;44:e214-8. [Crossref] [PubMed]
- Shintani Y, Inoue M, Kawamura T, et al. Multimodality treatment for advanced thymic carcinoma: outcomes of induction therapy followed by surgical resection in 16 cases at a single institution. Gen Thorac Cardiovasc Surg 2015;63:159-63. [Crossref] [PubMed]
- Momozane T, Inoue M, Shintani Y, et al. Trimodality Therapy for an Advanced Thymic Carcinoma With Both Aorta and Vena Cava Invasion. Ann Thorac Surg 2016;102:e139-41. [Crossref] [PubMed]
- Limmer S, Merz H, Kujath P. Giant thymoma in the anterior-inferior mediastinum. Interact Cardiovasc Thorac Surg 2010;10:451-3. [Crossref] [PubMed]
- Fujiwara A, Funaki S, Ose N, et al. Surgical resection for advanced thymic malignancy with pulmonary hilar invasion using hemi-clamshell approach. J Thorac Dis 2018;10:6475-81. [Crossref] [PubMed]
- Masaoka A, Ito Y, Yasumitsu T. Anterior approach for tumor of the superior sulcus. J Thorac Cardiovasc Surg 1979;78:413-5. [PubMed]
- Ohta M, Hirabayasi H, Shiono H, et al. Hemi-clamshell approach for advanced primary lung cancer. Thorac Cardiovasc Surg 2004;52:200-5. [Crossref] [PubMed]
- Shintani Y, Kanzaki R, Kawamura T, et al. Surgical resection for advanced lung cancer using the hemi-clamshell approach. Interact Cardiovasc Thorac Surg 2017;25:462-8. [Crossref] [PubMed]
- Shintani Y, Kanzaki R, Kusumoto H, et al. Pleuropneumonectomy for a large thymoma with multiple pleural dissemination using median sternotomy followed by posterolateral thoracotomy. Surg Case Rep 2015;1:75. [Crossref] [PubMed]
- Kondo K. Therapy for thymic epithelial tumors. Gen Thorac Cardiovasc Surg 2014;62:468-74. [Crossref] [PubMed]
- Attaran S, Acharya M, Anderson JR, et al. Does surgical debulking for advanced stages of thymoma improve survival? Interact Cardiovasc Thorac Surg 2012;15:494-7. [Crossref] [PubMed]


