
The clinical significance of serum adipocytokines level in patients with lung cancer
Introduction
Lung cancer is the highest morbidity and mortality malignant tumor in the world (1).The etiopathogenesis of lung cancer includes external environmental and inherent decisive factors including Family history, smoking, lifestyle, professional and dietary risk factors (2).Despite substantial advances in comprehensive diagnostic methods and therapeutic protocols in lung cancer, the 5-year survival rate was less than 20% because of the deficiency of early diagnosis and easy to recurrence and distant metastasis (3).Therefore, it is crucial to study early diagnosis biomarkers and explore new therapeutic modalities of lung cancer.
Obesity is related to an increased risk of developing multiple cancers, such as breast, prostate, endometrial cancer, and colon cancer (4-6). In obesity, dysfunctional adipose tissue causes metabolic disorders and a diminished ability of fat cells to store free fatty acids, as well as an increased secretion of different adipokines and cytokines which are known associated with tumor development (7). Adipocytes are the main components of adipose tissue, which was regarded as a site of maintain energy homeostasis by storing and mobilizing lipids during times of increased energy demand (8). Lipids are major components of the cell membrane and are essential for malignant tumors. Adipocyte-ovarian cancer cell co-culture identified up-regulation of fatty acid-binding protein 4 (a lipid chaperone) in ovarian cancer cells and promoted in vitro and in vivo tumor growth, suggesting adipocytes are an energy source of cancer cells (9). In adipocytes and lung cancer cells co-culture system, the results demonstrated a reciprocal interaction between adipocytes and lung cancer cells, which significantly enhanced lung cancer cell proliferation and metastasis (10). Adipocytes were also shown to promote tumor cells proliferation and the direct migration through interactions with certain cancer cells, including prostate, colon, and breast cancer (11-13). In addition, numerous studies have revealed that adipocytes are not only an effective source of energy but also secrete a variety of bioactive substances called adipocytokine, such as leptin, adiponectin, IL-6, VEGF, TNF-α, these secreted factors are able to influence tumor cell survival and behavior (7,14).
Adipocytokines regulate a variety of physiological and pathological processes by acting in autocrine, paracrine or endocrine manner. The accumulating evidence indicates that adipocytokines are associated with the development, progression and immune responses of several malignancies, and are attracting a lot of attention as potential tumor markers (15). In the tumor microenvironment, the binding of PD-1 and PD-L1 inhibits cytokine release and promotes tumor cell escaping from host immune attack (16). PD-L1 overexpression is associated with poor prognosis in non-small cell lung cancer (17). Although lung cancer is not considered to be an obesity-related disease (18), there is a complex interaction between adipose tissue, adipocytokines, insulin resistance and lung cancer pathogenesis (19-21). To date, the interaction between specific adipocytokines and the progression of lung cancer remains unclear and inconclusive. The aim of this study is to detect the expression of serum adipocytokines, explore their potential diagnostic ability and connection with clinicopathological features of lung cancer. In the present study, the serum levels of 12 obesity-associated adipocytokines were studied and we demonstrate that the serum levels of several of these adipocytokines correlated with the clinicopathological characteristics of lung cancer patients.
Methods
Study population
Forty-nine patients and twenty consecutive healthy controls treated in Department of The Second Affiliated Hospital of Anhui Medical University between July 2016 and May 2017 were enrolled into this prospective study. All cases were newly diagnosed and confirmed by pathological examination as lung cancer. The clinicopathological features (tumor size, pathological type, lymph node and distal metastasis) were determined by physical examination, pathological examination and imaging examinations. Patients who had received chemotherapy or radiotherapy and accompanying other malignancies were excluded from the study. The study was approved by the hospital ethical committee and all participants provided informed consent (approval number: PJ-YX2018-045).
We collected fasting whole blood from forty-nine patients and twenty healthy volunteers into a common centrifuge tubes or vacuum tubes (the blood collection tubes contain no anticoagulant, preservative or separating agent), stored at 4 °C for 30–45 min, centrifuge at 3,000–5,000 rpm/min for 10min, and take the supernatant for detection or stored at −80 °C for further analysis.
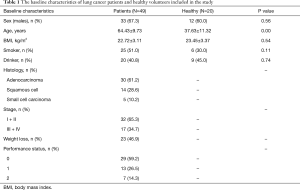
Among 49 patients with lung cancer there were 33 males, 16 females; median age: 64, range, 42–77 years. The sex and BMI in healthy controls were resemble to those in lung cancer patient groups, while there are significant differences in ages (P<0.01). Among all the patients were 25 (51.0%) with smoking history, 20 (40.8%) with drinking history and 23 (46.9%) with weight loss. Pathological examination revealed: 30 (61.2%) adenocarcinoma; 14 (28.6%) squamous cell; 5 (10.2%) small cell carcinoma.
Laboratory evaluation
The expression levels of adipocytokines in the fasting whole blood supernatants were measured by Human Obesity Array 3 Glass Slide box (RayBiotech, GA, USA). The experiment was performed according to the manufacturer’s instructions. Adipocytokines of different types were detected: the insulin-like growth factor binding protein-1 (IGFBP-1), tumor necrosis factor (TNFα), proangiogenic vascular endothelial growth factor (VEGF), TNF receptor I (TNF RI), resistin, TNF receptor II (TNF RII), brain-derived neurotrophic factor (BDNF), interleukin-6 (IL-6), plasminogen activator inhibitor-1 (PAI-1), leptin, IL-10, chemerin.
Cytokine assays
Take out the glass slide from the box, and let it air dry for another 1–2 h. Add 100 µL Sample diluent into each well and incubate at room temperature for 30 min to block slides. Decant buffer from each well. Add 100 µL of sample to each well. Incubate arrays at room temperature for 1–2 h, wash, decant the samples from each well, and wash 5 times (5 min each) with 150 µL of 1× Wash Buffer I at room temperature with gentle shaking, completely remove wash buffer. Reconstitute the detection antibody by adding 1.4 mL of sample diluent to the tube. Add 80 µL of the detection antibody cocktail to each well. Incubate at room temperature for 1–2 h, wash. After briefly spinning down, add 1.4 mL of Sample diluent to Cy3 equivalent dye-conjugated streptavidin tube, mix gently. Add 80 µL Cy3 equivalent dye-conjugated streptavidin to each well. Cover the device with aluminum foil to avoid exposure to light or incubate in dark room. Incubate at room temperature for 1 h, wash, remove water droplets completely, imaging. The raw data were analyzed with GSH-ADI-3 software (Agilent Technologies).
Statistical analyses
All the statistical analyses were performed using SPSS (Statistic Package for Social Sciences) 23.0 and GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Values are presented as the mean ± standard error of the mean from at least three independent experiments, Student’s t-test and Chi-square test were used for comparisons between two groups. The cytokine assay raw numerical data was analyzed by RayBiotech software, volcano plot was from the ggplot function. Using receiver operating characteristic (ROC) curves to estimate the diagnostic sensitivity and specificity of cytokine, differences between areas under the curves (AUCs) were assessed by the Wilcoxon test. All tests were two-sided; P<0.05 was considered as being statistically significant.
Results
Patients and healthy volunteers characteristics
The research summarizes the sex, age, body mass index (BMI) and history of past (history of smoking, history of drinking) of all study participants as well as clinical and pathological features of lung cancer patients (Table 1). Compared to patients, healthy cases presented a lower age (P<0.01), while there was no significant difference in BMI and previous history (including the smoking and drinking). Of 49 patients with lung cancer, 5 patients were diagnosed with small cell lung cancer and 44 with non-small cell lung cancer (30 had adenocarcinoma and 14 squamous cell carcinoma). Thirty-two patients (65.3%) were diagnosed with stages I and II, 17 patients (34.7%) stages III and IV, most patients had good performance status (PS) (29 with ECOG PS =0 and 13 with ECOG PS =1), while 23 out of 49 patients were classified as weight loss.
Full table
Comparison of adipocytokines levels in lung cancer patients and controls
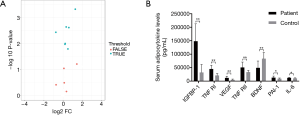
As shown in Table 2 and Figure 1, serum levels in the following 7 of the 12 adipocytokines assayed were found to be statistically different between patients and healthy volunteers. Significantly elevated serum levels of 6 adipocytokines (IGFBP-1, TNF RI, VEGF, TNF RII, PAI-1 and IL-6) (P<0.05) were found in lung cancer patients versus control subjects, whereas the serum BDNF levels were significantly lower in patients than in controls (P<0.01). In addition, no statistical difference was detected between the two groups concerning the serum levels of resistin, leptin, TNFα, IL-10 and chemerin.

Full table
Correlation between serum adipocytokine levels and clinicopathological features of lung cancer
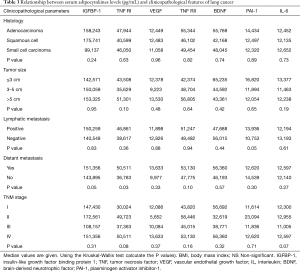
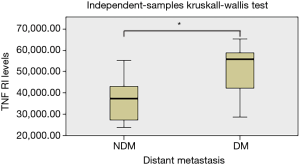
We further analyzed the possible association between serum adipocytokines levels and clinicopathological features of patients, and the results are summarized in Table 3. The statistical difference in adipocytokine serum levels were detected between groups of patients divided according to histology, tumor size, lymphatic and distant metastasis and TNM classification. Significantly higher levels of circulating TNF RI were found in patients with distant metastasis (P<0.05) (Figure 2). We did not find any correlation of the adipocytokines levels with tumor size and lymphatic metastasis of lung cancer patients. There was positive correlated tendency between several adipocytokines levels and patients in TNM classification, but no significant association was found (P>0.05).
Full table
Evaluation of diagnostic sensitivity and specificity of adipocytokines in lung cancer
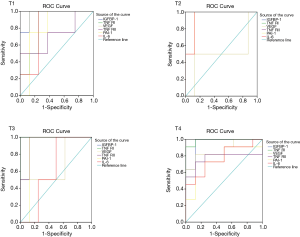
The ROC curves analysis of different activities cytokines is given in Figure 3. The area under the ROC curve of serum IGFBP-1, TNF RI, VEGF, TNF RII, PAI-1 and IL-6 for the diagnosis of lung cancer are 0.961, 0.961, 0.829, 0.803, 0.789 and 0.783 (95% CI: 0.880–1.041, 0.893–1.028, 0.672–0.986, 0.636–0.969, 0.590–0.989 and 0.592–0.974. P<0.05), the best cut-off point (according to Youden index) for circulating adipocytokines levels were 0.32, 0.83, 0.65, 0.72, 0.73 and 0.68 pg/mL, and the sensitivity and specificity of diagnosis of lung cancer were more than 70%.
Discussion
Lung cancer is the leading cause of cancer-related death in worldwide, usually with high metastasis rate and poor prognosis (1). Therefore, it is significant to research the effective biomarkers in diagnosis and prognosis of lung cancer. In the present study, we tested the expression levels of wide adipocytokines serum profile, explore its diagnostic potential and assess its clinical significance of newly diagnosed lung cancer patients. We demonstrated here that several circulating adipocytokines are elevated in the sera of lung cancer patients compared to controls, namely IGFBP-1, TNF RI, VEGF, TNF RII, BDNF, PAI-1 and IL-6, while BDNF were lower in patients. But only a few of these adipocytokines were of clinical relevance.
Adipocytokines are mainly synthesized and released by adipocytes and have various biological functions that regulate energy balance, lipid metabolism, and participate in inflammatory reactions (22). Numerous studies have demonstrated that adipocytokines are involved in the pathogenesis of several cancer types, including breast cancer (23), prostate cancer (24) and ovarian cancer (9). Moreover, there is accumulating evidence manifest the associativities between adipocytokines and lung cancer, such as leptin and adiponectin which are the most abundant adipocytokines secreted by adipose tissue. Many experts believed that high expression levels of leptin in serum and tissue of tumor patients is considered to promote tumor growth and progression (25-27), especially in lung cancer (20), but these research findings are inconsistent. In my study the leptin levels increase in the patients are not found. The same results were obtained in serum resistin, TNFα, IL-10 and chemerin levels, this may be due to the non-negligible heterogeneity of the studied patients.
By comparing the serum concentrations of adipocytokines between lung cancer patients and healthy controls, the results showed that circulating IGFBP-1, TNF RI, VEGF, TNF RII, PAI-1 and IL-6 presents a significantly elevated in patients with lung cancer, while BDNF were lower in patients. Kimura et al. (28). have shown that BDNF and its receptor TrkB are highly expressed in small cell lung cancer tissues, promote cell proliferation and migration through the BDNF/TrkB signaling pathway and that high expression is related to poor prognosis. Although it is not consistent with our experimental results, we also pointed out that the expression of BDNF in small cell lung cancer was higher than non-small cell lung cancer. The difference may be due to only a spot of patients with small cell lung cancer which were enrolled in this trial and more samples are needed in the future.
Besides, we further analyzed the relationship between these differential cytokines and the clinicopathological features of lung cancer patients, including pathological type, tumor size, lymph node and distant metastasis, and TNM stage. But only TNF RI was found clinically relevant, the expression of TNF RI was positively correlated with distant metastasis, the higher values of TNF RI in patients with advanced lung cancer. This means that TNF RI may involve in the development, progression and metastasis of lung cancer.
Metastasis is a complex process and the main cause of cancer-related deaths, especially in lung cancer. It is important to determine the metastasis related markers and potential molecular mechanisms for improving the prognosis of patients with lung cancer. It is well known that TNF-α is one of the factors regulating tumor microenvironment, which has both pro- and anti-tumoral effects through two major receptors (TNF RI and TNF RII) and play important roles in angiogenesis, apoptosis, inflammation and immune response (29). In addition, cytokines that associated with inflammatory responses were usually those most frequently correlated to clinicopathological features of cancers. Our study of the relationship between TNFR1 and the clinicopathological features of lung cancer is in accordance with this view. Studies on the cell level points out the expression of TNF RI can promote carcinogenesis. TNF RI directly promotes breast cancer cells proliferation by activating p42/p44 MAPK, JNK, PI3-K/Akt pathway (30). On the other hand, TNF RI enhanced cell death induction by CD95L and attenuated the poptotic effect of TRAIL (31). Moreover, TNF RI is also involved in the metastasis of many malignancies. Charles’s experimental results showed that TNF RI promotes peritoneal metastasis of mice ovarian cancer (32). In a colon tumor model, TNF RI-deficient mice cause a reduction in liver metastasis (33). In a word, TNF RI plays an important role in tumor growth and metastasis. Similarly, our experimental results also show that TNF RI is significantly elevated in the serum of lung cancer patients with distant metastasis compared to patients without distant metastases, which means TNF RI may involve in the metastasis of lung cancer and represents a poor prognosis of patients.
The key finding of the research is that the ROC curves point out serum IGFBP-1, TNF RI, VEGF and TNF RII, which present the high sensitivity and specificity, with AUCs of 0.962, 0.961, 0.829 and 0.803, respectively. Diagnostic accuracy of PAI-1 and IL-6 was lower with AUCs of 0.798 and 0.783 (P<0.05, Figure 2). In the present study population, there are newly diagnosed and not accompany other diseases and malignancies in all cases. Therefore, it is a good diagnostic method to detect serum levels of these cytokines for newly diagnosed lung cancer patients.
In summary, our study provides evidence that serum levels of several obesity-related cytokines are increased in lung cancer patients (namely IGFBP-1, TNF RI, VEGF, TNF RII, PAI-1 and IL-6), suggest that these cytokines play an important role in the occurrence and development of lung cancer. Moreover, serum levels of TNF RI were correlated to distant metastases of lung cancer and represents a poor prognosis of patients, may be a new target for lung carcinoma therapy. In addition, current results suggest that IGFBP-1, TNF RI, VEGF and TNF RII probably provide certain reference value for early diagnosis of lung cancer.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81402427), the Provincial Natural Science Foundation of Anhui (No. 1408085QH165) and Doctoral Specialty Foundation of Colleges and Universities (No.20133420120001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the hospital ethical committee (approval number: PJ-YX2018-045) and all participants provided informed consent.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Akhtar N, Bansal JG. Risk factors of Lung Cancer in nonsmoker. Curr Probl Cancer 2017;41:328-39. [Crossref] [PubMed]
- Chalela R, Curull V, Enríquez C, et al. Lung adenocarcinoma: from molecular basis to genome-guided therapy and immunotherapy. J Thorac Dis 2017;9:2142-58. [Crossref] [PubMed]
- Slattery ML, Curtin K, Wolff RK, et al. Diet, physical activity, and body size associations with rectal tumor mutations and epigenetic changes. Cancer Causes Control 2010;21:1237-45. [Crossref] [PubMed]
- Macciò A, Madeddu C, Gramignano G, et al. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. J Mol Med (Berl) 2010;88:677-86. [Crossref] [PubMed]
- Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, et al. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta 2011;1807:664-78. [Crossref] [PubMed]
- Morris EV, Edwards CM. Bone Marrow Adipose Tissue: A New Player in Cancer Metastasis to Bone. Front Endocrinol (Lausanne) 2016;7:90. [Crossref] [PubMed]
- Nieman KM, Romero IL, Van Houten B, et al. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta 2013;1831:1533-41. [Crossref] [PubMed]
- Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011;17:1498-503. [Crossref] [PubMed]
- Li FF, Zhang H, Li JJ, et al. Interaction with adipocytes induces lung adenocarcinoma A549 cell migration and tumor growth. Mol Med Rep 2018;18:1973-80. [PubMed]
- Laurent V, Guérard A, Mazerolles C, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun 2016;7:10230. [Crossref] [PubMed]
- Amemori S, Ootani A, Aoki S, et al. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol Gastrointest Liver Physiol 2007;292:G923-9. [Crossref] [PubMed]
- Tan J, Buache E, Chenard MP, et al. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol 2011;55:851-9. [Crossref] [PubMed]
- Caers J, Deleu S, Belaid Z, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia 2007;21:1580-4. [Crossref] [PubMed]
- Ntikoudi E, Kiagia M, Boura P, et al. Hormones of adipose tissue and their biologic role in lung cancer. Cancer Treat Rev 2014;40:22-30. [Crossref] [PubMed]
- Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006;203:883-95. [Crossref] [PubMed]
- Okita R, Maeda A, Shimizu K, et al. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol Immunother 2017;66:865-76. [Crossref] [PubMed]
- Yang Y, Dong J, Sun K, et al. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer 2013;132:1162-9. [Crossref] [PubMed]
- Boura P, Loukides S, Grapsa D, et al. The diverse roles of adiponectin in non-small-cell lung cancer: current data and future perspectives. Future Oncol 2015;11:2193-203. [Crossref] [PubMed]
- Petridou ET, Sergentanis TN, Antonopoulos CN, et al. Insulin resistance: an independent risk factor for lung cancer? Metabolism 2011;60:1100-6. [Crossref] [PubMed]
- Tong X, Ma Y, Zhou Q, et al. Serum and tissue leptin in lung cancer: A meta-analysis. Oncotarget 2017;8:19699-711. [Crossref] [PubMed]
- Karastergiou K, Mohamed-Ali V. The autocrine and paracrine roles of adipokines. Mol Cell Endocrinol 2010;318:69-78. [Crossref] [PubMed]
- Choi J, Cha YJ, Koo JS. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog Lipid Res 2018;69:11-20. [Crossref] [PubMed]
- Uehara H, Takahashi T, Oha M, et al. Exogenous fatty acid binding protein 4 promotes human prostate cancer cell progression. Int J Cancer 2014;135:2558-68. [Crossref] [PubMed]
- Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res 2004;10:4325-31. [Crossref] [PubMed]
- Uddin S, Bu R, Ahmed M, et al. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol Cancer 2009;8:74. [Crossref] [PubMed]
- Nyante SJ, Gammon MD, Kaufman JS, et al. Common genetic variation in adiponectin, leptin, and leptin receptor and association with breast cancer subtypes. Breast Cancer Res Treat 2011;129:593-606. [Crossref] [PubMed]
- Kimura S, Harada T, Ijichi K, et al. Expression of brain-derived neurotrophic factor and its receptor TrkB is associated with poor prognosis and a malignant phenotype in small cell lung cancer. Lung Cancer 2018;120:98-107. [Crossref] [PubMed]
- Ham B, Fernandez MC, D'Costa Z, et al. The diverse roles of the TNF axis in cancer progression and metastasis. Trends Cancer Res 2016;11:1-27. [PubMed]
- Rivas MA, Carnevale RP, Proietti CJ, et al. TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp Cell Res 2008;314:509-29. [Crossref] [PubMed]
- Rauert H, Stühmer T, Bargou R, et al. TNFR1 and TNFR2 regulate the extrinsic apoptotic pathway in myeloma cells by multiple mechanisms. Cell Death Dis 2011;2:e194. [Crossref] [PubMed]
- Charles KA, Kulbe H, Soper R, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest 2009;119:3011-23. [Crossref] [PubMed]
- Brodt P. Surviving host innate immunity: Cancer cells can turn a deadly assault into an advantage. Oncoimmunology 2012;1:1601-3. [Crossref] [PubMed]


