Prognostic significance of the pattern of pathological N1 lymph node metastases for non-small cell lung cancer
Introduction
In contrast to the TNM classification schemes for many other types of cancer, the classification of lymph node metastases in non-small cell lung cancer (NSCLC) relies on anatomical location alone. Some investigations have suggested that the total number of affected lymph nodes or lymph nodes stations may more accurately reflect prognosis (1,2). Initial analyses performed by the International Association for the Study of Lung Cancer (IASLC) for its updated 8th edition of the TNM classification system also suggested that a more differentiated classification of nodal metastases is needed (3).
Although the IASLC analyses were based on an enormous volume of data, in many cases the data on number and location of affected lymph nodes was either missing or complete. Additionally, Japan—which provided nearly 60% of the data—uses a different lymph node map than much of the non-Asian world. In the Asian (Naruke map) classification for example lymph nodes along the inferior border of the main stem bronchus are classified as N1, while in Europe and North America they are classified as N2. For these reasons the IASLC ultimately decided against making any definitive changes to the N descriptor in its 8th edition update. Clinicians, however, are still urged to document the number of affected N1 and N2 lymph nodes and lymph node stations to provide data for future analyses (4).
Despite advances in positron emission tomography-computed tomography (PET-CT), ultrasound-guided endobronchial biopsy, and video assisted biopsy, the clinical and pathological N statuses frequently disagree. It thus seems reasonable for any investigation of nodal disease to consider the cN und pN statuses separately. In this retrospective study of surgically treated NSCLC patients we restricted our analysis to patients with pathologically confirmed N1 disease and sought to determine whether those with multiple N1 metastases had worse outcomes than those with single N1 metastases.
Methods
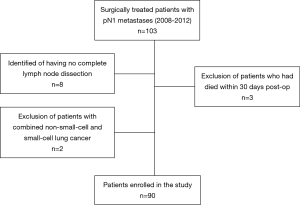
We retrospectively scanned the database of patients who had undergone surgical resection for NSCLC between 2008 and 2012 at the Heckeshorn Lung Clinic. For the sake of simplicity, we only considered patients with small tumors (pT1 or pT2 based on the 7th edition of TNM classification) and pathologically confirmed N1 status. We excluded patients where complete tumor resection had not been possible (not R0) and also patients where the lymph node data was not complete. Additionally, we excluded patients who had died within 30 days of surgical resection, in order to eliminate the potentially confounding factor of death due to immediate postoperative complications (Figure 1).
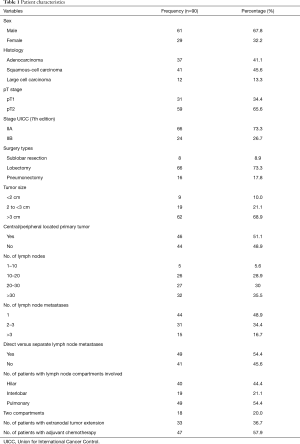
We identified 90 patients, who fit our criteria, and then extracted patient data on age, sex, tumor histology, infiltration of pulmonary and mediastinal lymph nodes, 5-year overall survival (OS), and 5-year disease-free survival (DFS).
The institutional review board waived the need for registration because all data had been gathered for internal quality control, and patients had already given their informed consent to have their data used for research purposes.
Patients were evaluated preoperatively by physical examination, bronchoscopy, chest radiograph, and FDG-PET/CT scans. Brain CT or magnetic resonance imaging (MRI) scans were only performed when clinical signs and symptoms suggestive of cerebral metastases were present.
All patients included in this study were treated in curative intent with lobectomy, pneumonectomy, or sublobar resection. In all cases systematic hilar and mediastinal lymph node dissection was performed according to standard practice (5). Thus, right-sided lung resections included dissection of the paratracheal, subcarinal, inferior mediastinal, interlobar, and hilar lymph nodes, and left-sided resections included dissection of aortic, infracarinal, inferior mediastinal, interlobar, and hilar lymph nodes. The pathologic specimens were assessed according to the IASLC map for patterns of tumor spread (6), and lobar (stations 12 and 13), hilar (station 10), and interlobar (station 11) lymph node metastases were classified as N1.
In most cases dissected lymph node stations were labeled by the surgeon, but the pathologist determined the lymph node status for the lobar stations (stations 12 and 13), as well as the total number of affected and non-affected N1 and N2 lymph nodes postoperatively.
For this investigation we retrospectively established a subcategory that differentiated between patients with multiple N1 metastases and those with a single N1 metastasis. We established a further subcategory that differentiated between cases in which hilar and lobar lymph node involvement was due to direct extension of the tumor and those in which the involvement was due to metastasis (with no direct connection to the primary tumor). In these cases, the distance between the nodal metastases and the primary tumor was coded in the preoperative CT scan. Cases with lymph node involvement due to both direct extension and metastasis were assigned the category of metastatic disease. Finally, we scanned pathology reports for patients with N1 nodal metastases that extended beyond the lymph node capsule, and looked at whether they had worse prognoses than patients with N1 metastases that did not extend beyond the capsule.
Statistical analysis
All data were analyzed using NCSS 12 Statistical Software 2018, (NCSS LLC. Kaysville, Utah, USA, ncss.com/software/ncss). Categorical variables were analyzed using the χ2 test and Fischer’s exact tests. Continuous variables were analyzed using either the Student’s t-test (normally distributed) or the Mann-Whitney test (not normally distributed). We used the Kaplan-Meier method to determine probability of survival (7), with the date of lung cancer surgery as the starting point and 5-year OS and DFS as end points. We used the log-rank test to analyze differences between subgroups, and we used Cox’s proportional hazards model to evaluate incremental risk factors influencing survival (8). Variables with a significance level of P<0.2 in the univariate survival analysis were included in the multivariate analysis. Probability values of less than 0.05 were considered statistically significant.
Results
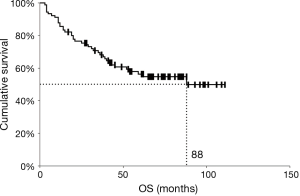
The last follow-up date included in this analysis was October 1st, 2018, and the median follow-up period for surviving patients (censored patients) was 74 months (range, 3–111 months). Among 90 patients who had N1 disease, 39 patients died within the follow-up period and 22 patients were lost to follow-up with no up-to-date information available. The 5-year OS rate was 56.3%, and the median survival time was 88 months (Figure 2). Patient demographics are summarized in Table 1. A median of 10 N1 lymph nodes (range, 3–50) and a median of 13 N2 lymph nodes (range, 3–35) were resected per patient.
Full table
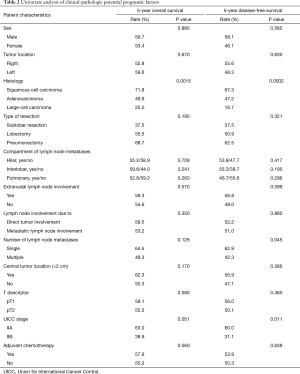
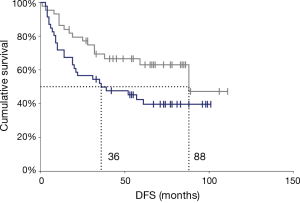
Results from the univariate analysis are presented in Table 2. Here, patients with large-cell carcinomas and higher UICC stage (IIB vs. IIA) had worse OS and DFS. Patients with multiple N1 metastases had worse DFS than patients with single N1 metastases, but for OS this relationship was not significant (Figure 3). We found no significant differences in either OS or DFS for the other variables investigated: age (≤65 vs. >65 years), sex, tumor location (right vs. left or central vs. peripheral), compartment with lymph node metastases, metastatic spread beyond the lymph node capsule vs. no extra-nodal spread, N1 due to direct tumor spread vs. due to metastasis, higher T indicator (pT1 vs. pT2), and adjuvant standard chemotherapy vs. no adjuvant chemotherapy (Table 2).
Full table
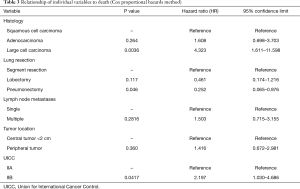
In the Cox multivariate analysis, we found that large-cell carcinoma, compared to other histological types (P=0.0036, hazard ratio: 4.323, 95% confidence interval: 1.611–11.598), and UICC stage IIB cancer (P=0.0417, hazard ratio: 2.197, 95% confidence interval: 1.030–4.686) was associated with a worse OS prognosis. Pneumonectomy, compared to more conservative resections, was associated with a better prognosis (P=0.046, hazard ratio: 0.252, 95% confidence interval: 0.065–0.976) (Table 3).
Full table
Discussion
The primary finding of this study, that single N1 lymph node metastases are not associated with a better OS than multiple N1 metastases, is somewhat surprising. In the univariate analysis patients with multiple N1 lymph nodes did in fact have a lower rate of 5-year DFS than those with a single N1 metastasis (42.3% vs. 62.9%), but in the multivariate analysis this association was not significant. This may suggest that we were simply underpowered and that this relationship was outweighed by the much stronger association between prognosis and histological type.
At the same time, although the existing literature suggests that greater overall burden of lymph node metastases is associated with a worse prognosis (1,2,9,10), the relationship is far from clear. The IASLC data used for the 8th edition demonstrated that patients with a single station of N1 metastases had better 5-year OS than those with multiple stations of N1 metastases, but this analysis was limited by missing data and variation in the lymph node maps used (4). While Wei et al. found an association between greater number of metastatic lymph nodes and worse outcome, this effect was much stronger for N2 nodes than for N1 nodes (1). Lee et al. found that a greater number of nodal metastases was associated with worse outcome, but the study did not differentiate between N1 and N2 nodes (11). Kang et al., in contrast, found that the number of metastatic nodes was not associated with a worse prognosis but that the number of affected nodal stations was (12). Interestingly, these studies all drew from largely Asian populations, treated in Asian hospitals. This is significant because of the differences in lymph node maps used in Asia and elsewhere. Additionally, certain cancer biological genetic differences, which needs further to be investigated, may exist as well.
Regarding the other N1 subcategories investigated, there is some evidence that N1 nodal disease from direct tumor extension has a better prognosis than N1 nodal disease from metastasis (13,14). In our analysis we only observed a non-significant trend in this direction, but here too we may simply have been underpowered. Our finding that extracapsular extension of lymph node metastases was not significantly associated with worse prognosis was only somewhat surprising. Although extracapsular extension seems to negatively influence outcome in breast cancer (15), malignant melanoma (16), and head and neck carcinoma (17) it currently has no place in the TNM classification for NSCLC. Moreover, although some studies suggest that it is associated with a worse prognosis in NSCLC (18), others—in concordance with or finding—report no association (19).
There is a high degree of variation in how resected lymph nodes are labeled and counted. This makes accurate lymph node analysis difficult and is an important limitation of both our study and others. Additionally, nodal classification is highly dependent on the information provided by the surgeon as well as on the dissection approach employed by the pathologist. International differences between lymph node maps have already been discussed, but as El-Sherief et al. have demonstrated, even within the same geographical region the IASLC lymph node map is frequently used both inconsistently and inaccurately (20). The right-sided boarder between lymph node stations 4 and 10, for example, is particularly problematic. An affected lymph node labeled as station 4 leads to a diagnosis of N2, while a lymph node labeled as station 10 leads to a diagnosis of N1. If the azygos vein is not dissected, however, correct classification can be difficult.
Additionally, fragmentation of lymph nodes during dissection can lead to an erroneously high number of metastases. For technical reasons N1 lymph nodes—the focus of our study—may be particularly prone to fragmentation and incorrect quantification. While mediastinal nodes are generally removed in a packet with the surrounding fatty tissue, N1 lymph nodes must be dissected from the narrow space of the hilum and from within the lung tissue. This highlights the need for surgeons to make every effort to avoid fragmentation of harvested lymph nodes and to be especially vigilant in labeling correctly. Moreover, it highlights the need for pathologists to follow a standardized and consistent protocol for dissecting and quantifying affected lymph nodes. Finally, it is important to consider how many lymph nodes and lymph node compartments need to be dissected intraoperatively to permit accurate assessment of nodal status. The IASLC currently recommends intraoperative dissection of at least three N1 nodes and three N2 nodes, as well as postoperative dissection of pulmonary specimens to search for intralobar metastases (21). It is unclear, however, whether this is sufficient.
Our secondary finding, that patients with large cell carcinoma have a worse prognosis than patients with other NSCLC histologic types, is in concordance earlier findings (22,23). This is unsurprising, as large-cell carcinoma is characterized by poorer differentiation—neither truly squamous nor glandular—and in other types of cancer as well, poorer differentiation is associated with poorer prognosis.
Interestingly, the T descriptor (pT1 vs. pT2) was not significant in this study, but the UICC stage (IIA vs. IIB) was, both for OS and for DFS. Since all patients in this analysis had pN1 status, the critical difference seems to be in the T descriptors: pT2a (>3–5 cm) and pT2b (>5–7 cm) (based on the 7th edition). Although we did not recode the T descriptors to align with the 8th edition, in most cases this difference would have been reflected in the 8th edition as the difference between pT2 (>3–5 cm) and pT3 (>3–5 cm) status and thus the difference between stages IIB and IIIA.
Interestingly, patients treated with pneumonectomies had the best outcomes (OS and DFS), and patients treated with sublobar resections had the worst outcomes. This finding, however, must be viewed with caution. Luzzi et al. found that pneumonectomy offered better locoregional control in patients with pN1 disease (24), but other investigations found that patients with lobectomies had better outcomes (25,26). Moreover, it is important to consider possible biases in patient selection. Candidates for pneumonectomy require better overall fitness and lung function than candidates for lobectomy. Since lobectomy, when possible, is the standard practice in our clinic, patients undergoing mere sublobar resections are generally limited by serious comorbidities such as advanced pulmonary emphysema or heart disease. Although these comorbidities likely have an independent detrimental effect on long term survival, we did not control for them as this was not the primary focus of our investigation. Furthermore, the better outcome for patients with pneumonectomies may be related to the fact that we excluded those who died within 30 days of surgery, which may have played more of a role in these patients than in lobectomy patients. Due to the enormous volume loss that pneumonectomy entails, a recommendation to perform this procedure for peripheral tumors cannot be recommended.
Our observations must be interpreted in the context of our study’s potential limitations. Here it is important to consider the retrospective nature of the study, as well as the complexity of the classification proposal for N1 disease. Since the study period, practice patterns have changed, as the prevalence of minimally invasive surgical modalities for N1 metastatic disease has increased. Within the study period all surgical resections were performed with an open thoracotomy approach. Additionally, we acknowledge that given the small sample size, this study may be underpowered for detecting all potential prognosticators between nodal status and survival. Thus, our findings will require validation in larger cohorts. Furthermore, given that standard helical CT imaging was used for follow-up to evaluate the patterns of failure after surgery, it is not clear, how these results will apply to other imaging modalities such as FDG-PET/CT.
In summary it seems clear that future classification systems for NSCLC could be improved by a more specific N indicator. Although we did not observe significant prognostic differences for long-term survival between single and multiple metastases in N1 disease, other studies may report different findings. The need for a standardized map as well as standardized practices among surgeons and pathologists will continue to remain of critical importance. A careful assessment of mediastinal, hilar, and interlobar lymph node involvement is always warranted. Moreover, future updates of the TNM staging system should incorporate more quality criteria recommendations that are both logical and easy to use in clinical routine. Only then will it be possible to generate reliable data that can be compared and incorporated into larger multi-center clinical trials.
Acknowledgments
The authors would like to thank Ms. S. Krueger for technical assistance and data processing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The institutional review board waived the need for registration because all data had been gathered for internal quality control, and patients had already given their informed consent to have their data used for research purposes.
References
- Wei S, Asamura H, Kawachi R, et al. Which is the better prognostic factor for resected non-small cell lung cancer: The number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol 2011;6:310-8. [Crossref] [PubMed]
- Saji H, Tsuboi M, Shimada Y, et al. A proposal for combination of total number and anatomical location of involved lymph nodes for nodal classification in non-small cell lung cancer. Chest 2013;143:1618-25. [Crossref] [PubMed]
- Bierley JD, Gospodarowicz MK, Wittekind C. editors. The TNM classification of malignant tumours. 8th edition. Hoboken, NJ: Wiley Blackwell, 2017.
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Dienemann H, Hoffmann H, Koebe HG. Technique and rationale of lymph node dissection in bronchial carcinoma. Chirurg 1998;69:412-7. [Crossref] [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958;53:457-81. [Crossref]
- Cox DR. Regression models with life-tables. J R Stat Soc Series B Stat Methodol 1972;34:187-220.
- Li ZM, Ding ZP, Luo QQ, et al. Prognostic significance of the extent of lymph node involvement in stage II-N1 non-small cell lung cancer. Chest 2013;144:1253-60. [Crossref] [PubMed]
- Li Q, Zhan P, Yuan D, et al. Prognostic value of lymph node ratio in patients with pathological N1 non-small cell lung cancer: a systematic review with meta-analysis. Transl Lung Cancer Res 2016;5:258-64. [Crossref] [PubMed]
- Lee JG, Lee CY, Park IK, et al. Number of Metastatic Lymph Nodes in Resected Non–Small Cell Lung Cancer Predicts Patient Survival. Ann Thorac Surg 2008;85:211-5. [Crossref] [PubMed]
- Kang CH, Ra YJ, Kim YT, et al. The Impact of Multiple Metastatic Nodal Stations on Survival in Patients With Resectable N1 and N2 Nonsmall-Cell Lung Cancer. Ann Thorac Surg 2008;86:1092-7. [Crossref] [PubMed]
- van Velzen E, Snijder RJ, De La Rivière AB, et al. Type of Lymph Node Involvement Influences Survival Rates in T1N1M0 Non-small Cell Lung Carcinoma: Lymph Node Involvement by Direct Extension Compared With Lobar and Hilar Node Metastases. Chest 1996;110:1469-73. [Crossref] [PubMed]
- Marra A, Hillejan L, Zaboura G, et al. Pathologic N1 non-small cell lung cancer: Correlation between pattern of lymphatic spread and prognosis. J Thorac Cardiovasc Surg 2003;125:543-53. [Crossref] [PubMed]
- Swaminathan S, Reintgen M, Kerivan L, et al. Extracapsular Extension in the Sentinel Lymph Node: Guidelines for Therapy. Clin Breast Cancer 2016;16:e65-8. [Crossref] [PubMed]
- Madu MF, Schopman JHH, Berger DMS, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol 2017;116:244-51. [Crossref] [PubMed]
- Puri SK, Fan CY, Hanna E. Significance of extracapsular lymph node metastases in patients with head and neck squamous cell carcinoma. Curr Opin Otolaryngol Head Neck Surg 2003;11:119-23. [Crossref] [PubMed]
- Luchini C, Veronese N, Nottegar A, et al. Extranodal extension of nodal metastases is a poor prognostic moderator in non-small cell lung cancer: a meta-analysis. Virchows Arch 2018;472:939-47. [Crossref] [PubMed]
- Suemasu K, Naruke T. Prognostic Significance of Extranodal Cancer Invasion of Mediastinal Lymph Nodes in Lung Cancer. Jpn J Clin Oncol 1982;12:207-12.
- El-Sherief AH, Lau CT, Obuchowski NA, et al. Cross-Disciplinary Analysis of Lymph Node Classification in Lung Cancer on CT Scanning. Chest 2017;151:776-85. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer NCCN.org NCCN Guidelines for Patients ® available at NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ®) 2018. Available online: (accessed January 1, 2019).www.nccn.org/patients
- Moumtzi D, Lampaki S, Zarogoulidis P, et al. Prognostic factors for long term survival in patients with advanced non-small cell lung cancer. Ann Transl Med 2016;4:161. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Nakatani Y, et al. Pulmonary Large Cell Neuroendocrine Carcinoma: Its Place in the Spectrum of Pulmonary Carcinoma. Ann Thorac Surg 2007;84:702-7. [Crossref] [PubMed]
- Luzzi L, Voltolini L, Campione A, et al. Pneumonectomy vs lobectomy in the treatment of pathologic N1 NSCLC: could the type of surgical resection dictate survival? J Cardiovasc Surg (Torino) 2003;44:119-23. [PubMed]
- Spaks A, Kopeika U, Pirtnieks A, et al. Long-term survival after lobectomy and pneumonectomy in patients with stage II non-small cell lung cancer (NSCLC). Lung Cancer 2012;77:28-43. [Crossref]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of Morbidity, 30-Day Mortality, and Long-Term Survival After Pneumonectomy and Sleeve Lobectomy For Non–Small Cell Lung Carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]