
Evaluation of a heparin monitoring protocol for extracorporeal membrane oxygenation and review of the literature
Introduction
Extracorporeal membrane oxygenation (ECMO) is a circulatory support technique used to provide respiratory and cardiac support. Data collected over recent years show a significant growth in the application of this complex, life-saving measure (1). It is important to evaluate protocols used to provide consistent ECMO support because of the elevated frequency of adverse events historically associated with it. Bleeding and thrombosis are important and prevalent risks in patients on ECMO support; these events can result from the activation of procoagulant and anticoagulant factors when a cannula makes contact with the endothelial surface of the blood vessels. These complications of ECMO are associated with a significant increase in mortality risk (2). Therefore, the Extracorporeal Life Support Organization (ELSO) anticoagulation guideline recommends the use of antithrombotic therapy during ECMO, but the guidelines leave it up to individual institutions to develop their own titration strategy (3).
Heparin is the most commonly used anticoagulant in ECMO (3). Historically, activated clotting time (ACT) was used to determine the therapeutic effect of heparin anticoagulation during ECMO. Therapeutic range is defined as 180–220 seconds. The ACT can be influenced by multiple factors, including platelet count, urine output, fibrinogen level, body temperature, hemodilution, other coagulation factor deficiencies, and renal replacement therapy (3,4). Therefore, some institutions have changed their protocol so as not to rely solely on the ACT to determine coagulation status during ECMO.
One alternative measure, activated partial thromboplastin time (aPTT), is an assay commonly used to determine the degree of heparin-induced anticoagulation. The aPTT measures the time to onset of fibrin formation in platelet-poor plasma (3). The aPTT is subject to interference from high levels of factor VIII and other constituents like alpha-2-macroglobulin.
Another measurement option is thromboelastography (TEG), a whole-blood test that quantifies the properties of clot formation and the integrity of the coagulation cascade at multiple phases. The TEG captures all in vivo coagulation except influences from endothelial effects. Thus, TEG can provide information about multiple phases of coagulation and may be more representative of in vivo coagulation than aPTT. Its use in ECMO is relevant, given that multiple factors can contribute to coagulation abnormalities (3).
Current ELSO guidelines for anticoagulation during ECMO recommend an initial heparin infusion rate of 7.5–20.0 units/kg/h (3). With this large range and little guidance regarding which laboratory tests to monitor, many institutions have turned to literature and experience to develop their own heparin protocol for ECMO. A survey study found that anticoagulation management policies vary greatly by center (5), and another found that the use of an anticoagulation protocol is associated with a decrease in hemorrhagic complications (6).
Therefore, a heparin monitoring protocol that uses aPTT and TEG to adjust heparin dosing was developed at our quaternary academic teaching facility, because using a single method to monitor heparin is no longer recommended (3). The goal of this study was to assess the rate of bleeding and thrombotic complications before and after the implementation of this heparin monitoring protocol for patients on ECMO.
Methods
An Institutional Review Board (IRB)-approved retrospective chart review was performed of ECMO-supported patients at least 18 years old, from January 2016 to March 2017 for the pre-protocol group and March 2017 through December 2017 for the post-protocol group, at Baylor St. Luke’s Medical Center in Houston, TX. Patients were excluded if they never received heparin while on ECMO, had a previous diagnosis of heparin-induced thrombocytopenia, or were allergic to heparin. Data were obtained from an IRB-approved ECMO database and the patients’ electronic medical records. Because this study was retrospective, the IRB waived the consent requirement.
The Quadrox oxygenator and Rotaflow pump (Getinge Group, Gothenburg, Sweden) were used to provide ECMO support. Venovenous ECMO patients were most commonly cannulated with a bicaval dual-lumen catheter (Avalon Elite; Getinge Group). Venoarterial ECMO patients were most commonly cannulated peripherally in a bifemoral configuration.
Before the heparin monitoring protocol was implemented, providers would adjust heparin dosing according to the aPTT alone. Furthermore, there was no guidance regarding when to start anticoagulation, the goal aPTT, or the starting dosage of heparin. Per the heparin monitoring protocol, which was implemented as a quality improvement project to reduce the incidence of bleeding, thrombotic, and neurologic events, anticoagulation with heparin was started if major or minor bleeding was not present and if the first computed tomography (CT) scan of the head, done with a portable CT machine, was normal 12 hours after ECMO was initiated. If major or minor bleeding was present or the first CT head scan was abnormal at 12 hours, the patient would be reassessed at 12 hours to determine whether heparin should be started. The recommended starting dosage of heparin was 7.0 units/kg/h.
The aPTT was checked every 6 hours and TEG twice daily. Therapeutic goals were defined as an aPTT of 60–80 seconds (1.5–2.0× baseline), a TEG reaction (TEG-R) time 2–4× baseline with no fibrinolysis, an anti-Xa level of 0.3–0.7 units/mL, and an antithrombin III (ATIII) level >50%. Although other institutions preferentially use rotational thromboelastometry, our institution does not. If the aPTT or TEG was supratherapeutic, the heparin infusion would be reduced by 100 units/h. If both lab values were subtherapeutic, the heparin infusion would be increased by 100 units/h. If one value was subtherapeutic and the other therapeutic, the patient’s anti-Xa level would be measured. If it was therapeutic, heparin infusion would continue at the same rate; if the anti-Xa level was above or below therapeutic, the hematopathologist would make the clinical decision. With regard to ATIII supplementation, it was recommended whenever the ATIII level was <50% without evidence of therapeutic heparin effect. As part of the protocol, it was recommended that a hematopathologist and neurologist be routinely consulted on each case. Patients who developed heparin-induced thrombocytopenia were started on bivalirudin per hospital protocol, which was available as an order set with an aPTT goal of 55–90 seconds.
The primary endpoints of our study were the rates of bleeding and thrombotic events. Bleeding was classified as major or minor according to the ELSO guidelines (3). Major bleeding included a hemoglobin (Hgb) drop ≥4 g/dL, use of more than 2 units of packed red blood cells (pRBC) in 24 hours, bleeding at a critical site (gastrointestinal, pulmonary, intracranial), or bleeding requiring surgical intervention. One change was made to the ELSO definition of bleeding to account for Hgb drops that were simply due to critical illness and multiple blood draws. We used a threshold Hgb drop of ≥4 g/dL, instead of ≥2 g/dL as recommended in the ELSO guidelines (3), because this threshold was consistent with the anticoagulation policy that was already in effect at our institution. The second primary endpoint, thrombosis, was defined as thrombus formation anywhere in the circuit (whether or not it led to circuit replacement) (3), disseminated intravascular coagulation (DIC), myocardial infarction, ischemic stroke, pulmonary embolism, deep vein thrombosis, or any arterial thromboembolic event (7).
Secondary endpoints included time in the therapeutic range, defined as percentage of time within the therapeutic aPTT during the first 48 hours of ECMO; physician compliance with the protocol, which was defined as the physician making appropriate consultations (neurology and hematopathology); time to heparin initiation; average heparin dosage over 48 hours; time to therapeutic level (defined by either aPTT or TEG); intensive care unit length of stay; and mortality. A subgroup of patients who received ATIII while on ECMO was also evaluated.
Continuous data were reported as mean ± standard deviation (SD). Categorical data were compared by using the χ2 test; continuous data were compared by using a two-tailed t-test or its nonparametric analogue, the Wilcoxon rank-sum test, as appropriate. An alpha level of 0.05 was used to determine statistical significance. Data analysis was performed in Microsoft Excel.
Results
After screening for exclusion criteria, there were 72 patients in the pre-protocol group (mean age, 56±15 y; 63.9% male) and 51 patients in the post-protocol group (mean age, 60±12 y; 66.7% male). There were no statistically significant differences in baseline characteristics between the two groups (Table 1).
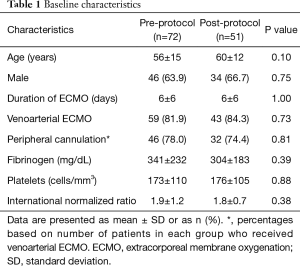
Full table
In the pre-protocol group, 69.4% of patients had major bleeding events, compared to 66.7% in the post-protocol group (P=0.85). The post-protocol group had significantly fewer retroperitoneal bleeds (15.3% vs. 2.0%; P=0.01) and a trend toward fewer pulmonary and central nervous system (CNS) bleeds (13.9% vs. 3.9%; P=0.07). The rate of minor bleeding events was similar in the two groups (8.3% vs. 9.8%; P=0.78). Thrombotic events occurred in 20.8% of the pre-protocol patients and in 27.5% of the post-protocol patients (P=0.39). Thrombotic events in the post-protocol group were principally related to circuit thrombosis and DIC. Fewer ischemic strokes occurred in the post-protocol group (5.6% vs. 2.0%; P=0.32). See Table 2 for other clinical outcomes.
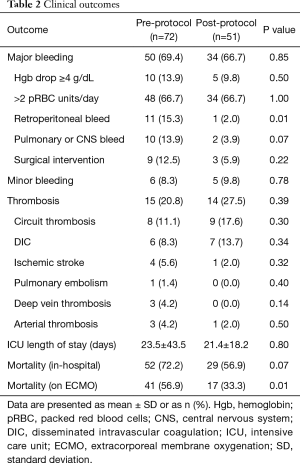
Full table
Patients in the pre-protocol group were in a therapeutic aPTT range 29% of the time, compared to 25% of the time in the post-protocol group (P=0.28) (Table 3). There was no difference in mean intensive care unit length of stay (23.5±43.5 vs. 21.4±18.2 days, P=0.80), but the post-protocol group had significantly lower mortality while on ECMO (56.9% vs. 33.3%, P=0.01). The mean heparin dosage was not significantly different between groups (10.1±5.0 vs. 9.4±5.1 units/kg/h, P=0.49). The rate of physician compliance with the protocol was 50.9%.

Full table
A subgroup of 29 patients who received ATIII while on ECMO was compared with 94 patients who did not receive ATIII. There was no statistically significant difference in average heparin dosage over 48 hours, aPTT time in therapeutic range, major bleeding, or minor bleeding (Table 4). However, the rate of thrombosis was higher in patients who received ATIII than in those who did not receive ATIII (48.2% vs. 15.9%; P<0.01). Furthermore, we found no significant difference between these two groups in time to therapeutic anticoagulation (14 vs. 18 h; P=0.08) or in the percentage of patients who never reached a therapeutic heparin level in 48 hours (14% vs. 12%; P=0.76).
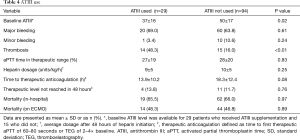
Full table
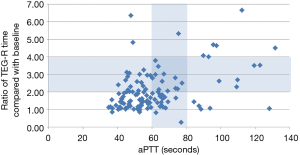
When comparing data points where both the TEG value and aPTT values were available at the same time point over 24 hours (n=43 with 117 data points), aPTT values were in the therapeutic range of 60–80 seconds 37% of the time when TEG-R times were therapeutic at 2–4× baseline. These values were more likely to be subtherapeutic than supratherapeutic in the first 24 hours after heparin administration began (Figure 1).
Discussion
A standardized anticoagulation-monitoring protocol using TEG and aPTT was shown to be safe and feasible. Our findings suggest that at our institution, initiating a heparin protocol for ECMO patients using modified ELSO criteria for major bleeding (Hgb decline of ≥4 gm/dL as opposed to ≥2 gm/dL as a defining metric) did not affect rates of major or minor bleeding or thrombosis. However, when Hgb decline and transfusion of pRBC >2 units were removed as measures of major bleeding, the post-protocol group had significantly less clinically relevant bleeding, including retroperitoneal, pulmonary, and CNS bleeding, and bleeding requiring surgical intervention (42% vs. 12%; P<0.01). Removing Hgb decline and transfusion requirements may be appropriate for ECMO patients, given that Hgb change is attributable to multiple factors, including circuit-related red blood cell clearance and hemodilution.
Patients with significantly delayed clot initiation per TEG (TEG-R time 2–4× normal) were in the aPTT-based therapeutic range only approximately 30% of the time. Figure 1 shows that when the TEG-R time indicated delayed clot initiation, the aPTT was in the 40- to 60-second range 50% of the time. This could explain why the percentage of time in therapeutic aPTT range was actually lower in the post-protocol group (25%) than in the pre-protocol group (29%), because the TEG was being used to guide dosing, as well. Several times, the TEG was therapeutic but the aPTT was subtherapeutic. Therefore, we collected a total of 42 anti-Xa levels from 14 patients. In most instances, the anti-Xa level returned to <0.3 units/mL, further indicating that less-intensive anticoagulation may be appropriate for ECMO patients. The hematopathologist involved with the case made the decision regarding heparin dose adjustment in these cases, usually erring on the conservative side and keeping the heparin drip at the same rate because the patient’s TEG was in the therapeutic range. The less-intensive anticoagulation in the post-protocol patients might account for the significant difference seen in the pre- and post-protocol rates of clinically significant bleeding, but it did not translate to an increased rate of thrombosis. The rates of circuit thrombosis and DIC were numerically higher in the post-protocol group, but the ischemic stroke rate was only 2% (vs. 6% in the pre-protocol group). Similarly, Panigada et al. (8) reported that adjusting heparin dosing to maintain an aPTT ratio between 1.5 and 2.0 frequently resulted in a “flat-line” TEG. To maintain an R time value without heparinase of 16–24 min, heparin was titrated to a corresponding median aPTT ratio of 1.37. In a randomized controlled trial of aPTT-guided versus TEG-guided heparin dosing in venovenous-ECMO patients, heparin dosing was lower in the TEG group. Compared with the TEG patients, patients in the aPTT group tended to bleed more (albeit not significantly more), especially at surgical sites (9).
The optimal approach for anticoagulation in ECMO support remains uncertain. There is considerable variation in practice across centers (10). Recently, some centers managing pediatric patients reported a benefit from using target anti-Xa levels of 0.3–0.8 units/mL (11,12) to guide ECMO anticoagulation. However, data from the EOLIA trial, which included adults on venovenous ECMO, indicate that targeting an aPTT of 40–55 seconds or an anti-Xa level of 0.2–0.3 units/mL led to low rates of hemorrhagic stroke and acceptable rates of circuit exchange (28%) (13). Although anti-Xa level is the most specific measure of heparin effect, it is not affected by other potential changes in the coagulation system (e.g., decreased synthesis of coagulation proteins due to hepatic dysfunction). Thus, anti-Xa monitoring may be too specific a measure in patients at risk for complex coagulopathies. Table 5 reviews available literature comparing TEG-R time and anti-Xa to more classical monitoring parameters in relation to heparin dose. Across these studies, TEG-R time and anti-Xa tended to be more reliable monitoring parameters in relation to heparin dose, whereas ACT was the least reliable. One study found that during pediatric ECMO, ACT correlated poorly with anti-Xa activity, and the investigators concluded that ACT results should be interpreted with caution when managing anticoagulation (21). Another study of pediatric ECMO patients found that whereas ACT did not correlate well with heparin dosage (units/kg/h), aPTT was significantly correlated with it, albeit weakly (22). However, these studies were small, retrospective, and focused mainly on the pediatric population. Furthermore, many of the reports do not include important details (e.g., whether the anti-Xa reagent used endogenous or exogenous ATIII and time in therapeutic range) necessary for rendering a definitive position on anti-Xa as an ideal monitoring system. For this reason, we elected to use testing parameters that reflect a broader array of effects on coagulation. Among the more global tests, it is unclear whether aPTT or TEG better reflects heparin effect. Based on clinical findings, this analysis offers preliminary data to suggest that in the context of the acute-phase reactants as seen in ECMO patients, TEG-based anticoagulation may be a more clinically useful measure than aPTT. Similar findings suggesting the superiority of whole blood viscoelastic assays over aPTT alone in predicting bleeding have been reported in the trauma and surgical literature.
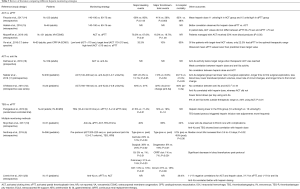
Full table
Our current study also raises the question of whether current anticoagulation practices in ECMO patients are too aggressive, leading to an increased risk of bleeding. Advances in technology, such as circuit coating with biocompatible surfaces, have reduced the incidence of circuit thrombosis. Recent studies of minimal anticoagulation, particularly in patients on venovenous ECMO (which poses a lower risk of clotting than venoarterial ECMO), associated this approach with a lower incidence of bleeding and few thrombotic events (6–23%) (9,23-25). In a study of Korean patients (n=29) who received no heparin for at least 3 days, Chung et al. (26) found that the patients had no intracardiac, intravascular, or intracircuit thrombotic complications while off heparin. Among our patients, the effects of heparin were more often subtherapeutic than supratherapeutic. Although our post-protocol thrombotic rate appears high at 28%, when DIC is removed from that definition, the thrombotic rate decreases to 22%, which is comparable to other reported findings (2,9,23-25,27).
In our study, although the pre- and post-protocol groups had similar transfusion requirements based on pRBC use, the post-protocol group trended toward less bleeding overall and had fewer clinically significant bleeds. Adopting a protocol allows more clearly defined anticoagulation management in these patients, which the ELSO guidelines recommend (3), and improves the team’s ability to assess protocol safety and efficacy. In addition, following a protocol reduces the variability in anticoagulation practices among individual clinicians. A potential major signal in this study was the significant difference between groups in survival on ECMO. Additional study of survival in ECMO-supported patients is essential. Outcomes could be further improved in the future: A formal order set was implemented in our institution’s electronic medical record after this study was completed, which may increase compliance and ensure that physicians order all necessary medications, laboratory tests, and consultations, as only 51% of patients in the post-protocol group received neurology and hematopathology consultations.
We conducted a subgroup analysis of 29 patients who received ATIII compared to 94 patients who did not receive ATIII while on ECMO. Per protocol, the goal ATIII level was >50%, but no formalized protocol was available to indicate when and how to dose ATIII. There was no statistically significant difference in heparin dosage, aPTT time in therapeutic range, or major or minor bleeding rate. The rationale for ATIII supplementation comes from recommendations from ELSO in 2009 (23) to maintain a normal ATIII level, and from the mechanism of action of heparin. Heparin binds to ATIII, which produces a conformational change that potentiates its effect, thereby increasing its inhibition of thrombin and other coagulation factors. Higher ATIII levels are associated with increased thrombin inhibition, but increasing ATIII levels may not sufficiently affect heparin resistance, given that access to heparin unbound to interfering substances is the primary problem. Also, increasing the heparin dosage may not have a therapeutic effect in heparin-resistant patients. When the ELSO guidelines were issued, no ECMO-related studies strongly supported ATIII supplementation (28), and it is still controversial today.
Similar to previous studies (28,29), our study found no significant difference in heparin dosage, time in therapeutic range, or bleeding events between patients on ECMO who received ATIII supplementation and those who did not. There was a significant difference in rate of thrombosis (48.2% vs. 15.9%, P<0.01), perhaps because these patients had a lower ATIII level at baseline, which can put the patient at higher risk for thrombosis. Our results further indicate that inadequate anticoagulation does not seem to contribute to this elevated rate of thrombosis. This is similar to Byrnes et al.’s finding that among patients on ECMO, circuit failure was more frequent in ATIII recipients than in nonrecipients (28), presumably because the ATIII recipients more often had clots within the circuit.
Mortality during ECMO support was significantly lower in the post-protocol group than in the pre-protocol group. This difference could be related to the post-protocol group’s lower rates of retroperitoneal, intracranial, and pulmonary bleeding. It could also be related to improvements in practice, surgical technique, and patient selection that were made over the study period and that therefore disproportionately affected the post-protocol patients.
Our study had limitations, including its retrospective design, inability to account for confounders to bleeding and thrombosis (i.e., other indications for blood transfusions or circuit replacement), and small sample size. Additionally, only 51% of the post-protocol cases had a hematopathologist and neurologist involved (which was the definition of compliance); having these services involved with more cases could have improved post-protocol bleeding and thrombotic outcomes. Additional studies with a larger sample or a prospective design are warranted to more fully assess bleeding and thrombosis rates in our institution’s ECMO patients.
Conclusions
A standardized anticoagulation-monitoring protocol using TEG and aPTT was shown to be safe and feasible. The rate of major bleeding—the primary study endpoint—as defined by ELSO parameters did not differ between the treatment groups. However, we observed a significant difference in the secondary endpoints of mortality and retroperitoneal bleeds, suggesting an important gain from the intervention and further demonstrating that care should be protocol driven. This also invites the question of whether we are over-anticoagulating our patients. We did observe that a lower baseline ATIII level may be placing patients at higher risk for thrombosis. Further study of the value of ATIII supplementation in ECMO patients is needed.
Acknowledgments
Stephen N. Palmer, PhD, ELS, contributed to the editing of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Data were obtained from an IRB-approved ECMO database and the patients’ electronic medical records. Because this study was retrospective, the IRB waived the consent requirement.
References
- Gerke AK, Tang F, Cavanaugh JE, et al. Increased trend in extracorporeal membrane oxygenation use by adults in the United States since 2007. BMC Res Notes 2015;8:686. [Crossref] [PubMed]
- Sy E, Sklar MC, Lequier L, et al. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: a systematic review and meta-analysis. J Crit Care 2017;39:87-96. [Crossref] [PubMed]
- The Extracorporeal Life Support Organization (ELSO). Extracorporeal Life Support Organization (ELSO) general guidelines for all ECLS cases. Available online https://www.elso.org/Portals/0/Files/elsoanticoagulationguideline8-2014-table-contents.pdf
- Perry DJ, Fitzmaurice DA, Kitchen S, et al. Point-of-care testing in haemostasis. Br J Haematol 2010;150:501-14. [Crossref] [PubMed]
- Bembea MM, Annich G, Rycus P, et al. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med 2013;14:e77-84. [Crossref] [PubMed]
- Northrop MS, Sidonio RF, Phillips SE, et al. The use of an extracorporeal membrane oxygenation anticoagulation laboratory protocol is associated with decreased blood product use, decreased hemorrhagic complications, and increased circuit life. Pediatr Crit Care Med 2015;16:66-74. [Crossref] [PubMed]
- Hickey M, Gatien M, Taljaard M, et al. Outcomes of urgent warfarin reversal with frozen plasma versus prothrombin complex concentrate in the emergency department. Circulation 2013;128:360-4. [Crossref] [PubMed]
- Panigada M, Iapichino G, L'Acqua C, et al. Prevalence of "flat-line" thromboelastography during extracorporeal membrane oxygenation for respiratory failure in adults. ASAIO J 2016;62:302-9. [Crossref] [PubMed]
- Panigada M, Iapichino GE, Brioni M, et al. Thromboelastography-based anticoagulation management during extracorporeal membrane oxygenation: a safety and feasibility pilot study. Ann Intensive Care 2018;8:7. [Crossref] [PubMed]
- Esper SA, Welsby IJ, Subramaniam K, et al. Adult extracorporeal membrane oxygenation: an international survey of transfusion and anticoagulation techniques. Vox Sang 2017;112:443-52. [Crossref] [PubMed]
- Niebler RA, Parker H, Hoffman GM. Impact of anticoagulation and circuit technology on complications during extracorporeal membrane oxygenation. ASAIO J 2019;65:270-6. [Crossref] [PubMed]
- O'Meara LC, Alten JA, Goldberg KG, et al. Anti-Xa directed protocol for anticoagulation management in children supported with extracorporeal membrane oxygenation. ASAIO J 2015;61:339-44. [Crossref] [PubMed]
- Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 2018;378:1965-75. [Crossref] [PubMed]
- Fitousis K, Klasek R, Mason PE, et al. Evaluation of a pharmacy managed heparin protocol for extracorporeal membrane oxygenation patients. Perfusion 2017;32:238-44. [Crossref] [PubMed]
- Atallah S, Liebl M, Fitousis K, et al. Evaluation of the activated clotting time and activated partial thromboplastin time for the monitoring of heparin in adult extracorporeal membrane oxygenation patients. Perfusion 2014;29:456-61. [Crossref] [PubMed]
- Mazzeffi MA, Tanaka K, Roberts A, et al. Bleeding, thrombosis, and transfusion with two heparin anticoagulation protocols in venoarterial ECMO patients. J Cardiothorac Vasc Anesth 2019;33:1216-20. [Crossref] [PubMed]
- Yie K, Chon SH, Na CY. Activated clotting time test alone is inadequate to optimize therapeutic heparin dosage adjustment during post-cardiopulmonary resuscitational extracorporeal membrane oxygenation (e-CPR). Perfusion 2016;31:307-15. [Crossref] [PubMed]
- Delmas C, Jacquemin A, Vardon-Bounes F, et al. Anticoagulation monitoring under ECMO support: a comparative study between the activated coagulation time and the anti-Xa activity assay. J Intensive Care Med 2018.885066618776937. [PubMed]
- Moynihan K, Johnson K, Straney L, et al. Coagulation monitoring correlation with heparin dose in pediatric extracorporeal life support. Perfusion 2017;32:675-85. [Crossref] [PubMed]
- Liveris A, Bello RA, Friedmann P, et al. Anti-factor Xa assay is a superior correlate of heparin dose than activated partial thromboplastin time or activated clotting time in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med 2014;15:e72-9. [Crossref] [PubMed]
- Bembea MM, Schwartz JM, Shah N, et al. Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J 2013;59:63-8. [Crossref] [PubMed]
- Maul TM, Wolff EL, Kuch BA, et al. Activated partial thromboplastin time is a better trending tool in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med 2012;13:e363-71. [Crossref] [PubMed]
- Agerstrand CL, Burkart KM, Abrams DC, et al. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg 2015;99:590-5. [Crossref] [PubMed]
- Krueger K, Schmutz A, Zieger B, et al. Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: An observational study in more than 60 patients. Artif Organs 2017;41:186-92. [Crossref] [PubMed]
- Yeo HJ, Kim DH, Jeon D, et al. Low-dose heparin during extracorporeal membrane oxygenation treatment in adults. Intensive Care Med 2015;41:2020-1. [Crossref] [PubMed]
- Chung YS, Cho DY, Sohn DS, et al. Is stopping heparin safe in patients on extracorporeal membrane oxygenation treatment? ASAIO J 2017;63:32-6. [Crossref] [PubMed]
- Sklar MC, Sy E, Lequier L, et al. Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure. A systematic review. Ann Am Thorac Soc 2016;13:2242-50. [Crossref] [PubMed]
- Byrnes JW, Swearingen CJ, Prodhan P, et al. Antithrombin III supplementation on extracorporeal membrane oxygenation: impact on heparin dose and circuit life. ASAIO J 2014;60:57-62. [Crossref] [PubMed]
- Niebler RA, Christensen M, Berens R, et al. Antithrombin replacement during extracorporeal membrane oxygenation. Artif Organs 2011;35:1024-8. [Crossref] [PubMed]


