
Hybrid operating room—one stop for diagnosis, staging and treatment of early stage NSCLC
Introduction
With gaining evidence of the efficacy of lung cancer screening with low dose computer tomography (CT) (1), more small, indeterminate lung nodules or ground glass opacities (GGO) are being discovered. However, these sub-centimeter lesions often harbor pre-malignant or early stage lung cancer, especially if solid component is present (2). The ability to obtain pathological diagnosis of these small lesions that are often peripheral through biopsy is important. Excision of these nodules with sublobar resection can provide diagnosis and at the same time treatment with excellent prognosis (3). The accurate localization and marking of these small lesions is important to allow rapid intra-operative identification of the lesion, for precision surgery to provide adequate resection margins and at the same time preserve lung parenchyma. The challenges are the ability to accurately reach these small lesions for either biopsy or lung lesion marking, associated with the least complications, given the limitations of percutaneous and endobronchial approaches via current bronchoscopic navigation platforms. Many of the workflow and localization methods can potentially be utilized and optimized within the hybrid operating room (HOR) setting with cone-beam CT scanning facility and full operating theatre capabilities (4). This article will provide a perspective of how the amalgamation of advanced imaging in the operative environment, endoscopy, localization techniques and surgery in the HOR can provide a one-stop strategy for diagnosis, staging and treatment of early stage non-small cell lung cancer (NSCLC).
Rationale and setting of the hybrid operating room
An important aim of the hybrid operating room is to provide a one-stop, cost effective procedural flow to enhance diagnostic accuracy and reduce complications associated with patient transfer. The success of the HOR depends on high resolution imaging, precise localization tools, accurate marking or sampling techniques, and effective treatment modalities. A list of examples in each aspect is summarized in Table 1. The first hybrid theatre for thoracic surgery, the Advanced Multimodal Image-guided Operating (AMIGO) suite, was established in Brigham and Women Hospital in 2013, utilizing fluoroscopy, CT, MRI, near-infrared and PET as imaging modalities (5). A relatively smaller suite was established in the authors’ institution, which is designed with a multi-disciplinary mindset to provide accurate localization with percutaneous CT-guided hookwire insertion, electromagnetic navigation bronchoscopy (ENB) and virtual bronchoscopic navigation tools (Figure 1). Staging procedures, mainly nodal staging, can be obtained by both endobronchial ultrasound (EBUS) or ENB. The HOR is capable of different treatment modalities including video-assisted thoracoscopic (VATS) procedures, thermal and cryoablative procedures.

Full table
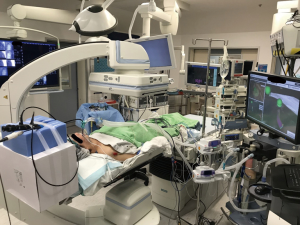
It is paramount to take into consideration the workflow of procedures to avoid time-consuming switching between different modalities. Highly flexible floor-mounted devices, movable roof-mounted screens and floatable angiography tables are common examples (6). During procedures, patient monitoring devices and the endotracheal tube should be arranged in a way not to interfere with imaging modalities, for instance the cone-beam CT rotation. If the suite is ENB or fluoroscopically enabled, the patient’s table and accessories should be designed to contain no magnetic interfering or radio-opaque parts.
Imaging techniques: cone-beam computer tomography (CBCT) in HOR
As opposed to traditional CT which are fan-beamed, rotating and obtaining a slice of image each time while patient is advanced, cone-beam CT rotates only once in 360 degrees to provide a volume of image without the need of patient movement. CBCT carries the benefits of lower cost, lower dose and more compact than fan-beamed CT. Most importantly, CBCT is able to provide excellent sub-millimeter spatial resolution combined with soft tissue visibility at a low radiation dose (~4.3 mGy/scan) (7). The average error of CBCT in visualizing target lesion and adjacent critical anatomy is less than 2 mm. In collapsed lung, only a slightly increased dose (up to 11.1 mGy) is needed to visualize target lesions, making it a potentially useful salvage tool in cases when the marking technique has failed to localize the lung lesion following one-lung ventilation (8). The availability of CBCT allows clinicians to choose the best technique for marking the lung nodule usually based on lesion location and expertise, but also for example whether there is presence of bronchus sign for ENB route, or lack of shielding of bony structures for the percutaneous hookwire route. The safety of a procedure can be ensured by CT visualization of adjacent important vessels to be aware of during marking or biopsy. In general, the CBCT is utilized in two stages: first to obtain a baseline imaging of the concerned region of lung for devising localization routes to the lung lesion, second to confirm the tip of biopsy or marking tools within target lesions.
Multi-disciplinary approach
The success of hybrid theatre for diagnosis, staging and treatment of pulmonary malignancies depends heavily on multi-disciplinary collaboration by surgeons, anesthesiologists, pulmonologists, radiologists and pathologists. Hookwire or microcoil placements in general are best performed by trained interventional radiologists, while expertise at frozen section or rapid on-site evaluation (ROSE) by pathologists is paramount to direct subsequent treatment and efficient use of the HOR (9). Very early staged tumours or pre-malignant lesions may be feasible for local ablative lesions or sublobar resection, whereas more advanced tumours would benefit from major anatomical lung resection (10). In case of insufficient material biopsied, ROSE allows for immediate re-biopsy until a diagnosis is achieved. Communication with the anesthesiologist is important for the optimizing ventilator setting for performing CBCT during ENB procedure; and in cases of hookwire localization coordinating the one-lung ventilation prior to resection, since pre-mature collapse of the lung can result in hookwire dislodgement (11).
Operative techniques
Localization tools: hookwire/microcoil
Percutaneous insertion of a hookwire or microcoil into target lesion under CT guidance has been a well-established method of pre-operative localization, especially useful for single ports VATS where limited access makes palpation of the lung challenging. In retrospective cohorts, the localization rate using this approach is up to 94% (12). Traditionally, the percutaneous hookwire/microcoil placement is performed in the radiology suite, followed by patient transferal to the conventional operative room for surgery. The prolonged time between localization and surgery, and the requirement of patient transferal, causes multiple drawbacks including patient discomfort, and increase risk of pneumothorax and dislodgement of localizing material. Up to 24% of patients suffer from pneumothorax after hookwire insertion, of which 2–4% require chest drain insertion before surgery. Approximately 2–10% experience wire dislodgement from the mounting pleura (12), making subsequent nodule localization extremely difficult.
Since 2013, our team has been performing percutaneous image-guided hookwire placement under CBCT guidance in the HOR, immediately followed by VATS resection for small lung lesions (Figure 2) (13). We completed 32 consecutive cases of sublobar resection of small pulmonary nodules (mean size of 9.1±4.6 mm) with this approach as of September 2017 (11). The hookwire placement is performed by interventional radiologist guided by CT and takes approximately 30 minutes. Interestingly, in our cohort 47% patients were detected on CBCT to have pneumothorax immediately after hookwire insertion but none required intervention. The average time interval between end of hookwire insertion and start of VATS surgery was 41.1±15 minutes, which was significantly shorter than the conventional approach of performing hookwire insertion and operation in separate suites. All the lesions were accurately marked and resected, and no wire dislodgement occurred. To mediate the consequences of hookwire dislodgement after lung deflation, options include injecting dye to the lesion through needle before threading in the hookwire, improving the design of hookwire tip, such as a spiral helix to provide better anchor (14), or salvage imaging by CBCT in a collapsed lung.
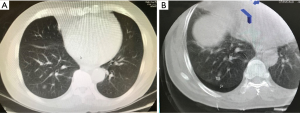
However, hookwire localization still has its limitations. Deep lesions that are >40 mm from the pleural surface is a relative contraindication as complications can be severe (15). In addition, it is often not feasible for hookwire to reach lesions at the apex/diaphragmatic or mediastinal regions, or areas shielded by the scapula.
Localization tools: electromagnetic navigation bronchoscopy (ENB)
The use of ENB as a diagnostic tool has been known for more than a decade, but its application for localizing small lung lesions in the HOR is relatively novel. The technology enables accurate navigation to peripheral lung lesions, using a virtual three-dimensional (3D) bronchial map made from a pre-operative CT scan using specialized software. An example of such system is SuperDimension Navigation System (Medtronic, Minneapolis, MN, USA), whereby a standard bronchoscope is inserted up to segmental bronchus, and a locatable electromagnetic guide (LEG) is advanced following the pre-planned navigational route until reaching the target. Subsequently, the LEG is removed leaving the extended working channel (EWC) through which tools can be passed to perform biopsy, dye-marking, and fiducial markers placement. When ENB is performed in the HOR, an advantage is that in appropriate cases, VATS lung surgery can proceed immediately afterwards with exchange of single lumen endotracheal tube for double-lumen tube, and turning of patient to lateral position (16).
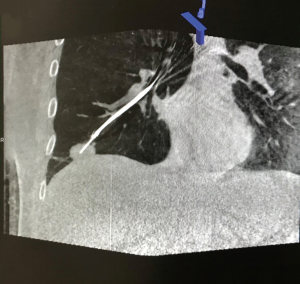
ENB studies based on earlier systems reported average fiducial target registration error of 9±6 mm for ENB (17), but the value is expected to be lower with more recent advanced versions. Retrospective studies reported excellent ENB marking success rate ranging from 79% to 100% (16). Reasons for unsuccessful markings are mainly due to extravasation of dye into pleural surface. Navigational error may be due to the torque from tools passed down the EWC, CT to body divergence or registration error, but a final check of needle position can always be ensured by a 6-second CBCT scan with ventilation suspension, and subsequent real time adjustment of EWC tool with image-guidance if necessary, thereby significantly improving the accuracy of ENB in a hybrid setting (Figure 3) (18).
Marking technique: triple contrast dye marking
Various marking methods have been devised throughout the development of VATS surgery, including radioisotope markers and different forms of dye, each has its own advantages and problems. The authors presented an innovative technique of triple contrast dye marking, along the concept of a “cocktail dye”, in order to have the best of all worlds (19). It consists of equal volumes of iohexol imaging contrast, methylene blue and indocyanine green (ICG), and an amount between 0.2–0.5 mL is injected into the target lung nodule, typically via ENB route. The iohexol contrast allows the dye to be clearly seen on fluoroscopy or CT, confirming correct position and adequate amount of dye injection. Methylene blue markings should be seen upon entering the pleural space during VATS under standard white light video thoracoscope. However, if the dye is injected deep in the parenchyma or if the lung is pigmented, methylene blue may not be visible. The video thoracoscope can then be switched to near infrared light, making the ICG brightly florescent, which can be seen up to as much as 2 cm from the lung surface (Figure 4).
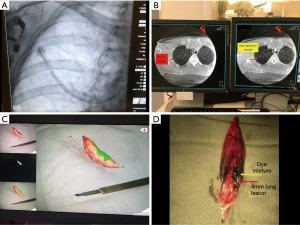
Triple dye marking of right upper lobe 4 mm lung lesion by ENB in HOR followed by VATS wedge resection. (A) Navigation and dye injection needle deployed based on CBCT iGuide (Siemens Healthineers, Germany) cross-marking with blush from iohexol contrast as lesion not visible on fluoroscopy, (B) injection needle position confirmed adjacent to lung lesion by CBCT, (C) VATS resected lung wedge marked by methylene blue (top left) and ICG fluorescence (main) seen through Pinpoint (Stryker, USA), (D) cut open specimen displaying the dye mixture (yellow arrow) relationship to the 4mm nodule (red arrow). ENB, electromagnetic navigation bronchoscopy; CBCT, cone-beam computer tomography; HOR, hybrid operating room.
Treatment modality: image-guided single port VATS (iSPVATS) and non-intubated single port VATS (NISPVATS)
Surgery remains the gold standard for biopsy proven early stage resectable lung cancer in patients with adequate lung function. Classically, VATS was performed with three ports to allow easier traction, dissection and visualization. With improvement in videoscopes and innovation of endostaplers which have slimmer profiles and flexible tips (20,21), single port VATS has emerged to be capable of major anatomical lung resections with equal margin clearance and efficacy, and the advantage of involving only one intercostal space and less pain (22-24). A problem with VATS, and perhaps more so in single port VATS, is the small access, instrument fencing and thus limited ability to palpate and localize small lung lesions (25). However, with adjuvant accurate localization of lung nodules enabled by the hybrid theatre, for instance ENB dye marking or hookwire localization, there is no longer a need for palpation of lung nodule through a small port (26,27). With precise localization of lung nodules, a simple small wedge resection is often adequate to treat pre-malignant or very early stage lung cancers (28).
There have been increasing interest in non-intubated VATS for sublobar resection, in an attempt to further improve overall outcome by avoiding the side effects of general anaesthesia and one-lung ventilation. A meta-analysis has shown that NIVATS can reduce operative morbidity and hospital stay compared to equivalent procedures performed under general anaesthesia, and some studies have even reported same day discharge (29-31). Image-guided non-intubated single port VATS in the hybrid theatre has great potential in providing a one-stop, minimally invasive workflow to expedite patient care (32).
Treatment modality: lung ablation
In patients with severe limiting comorbidities, inadequate lung function or refusal of surgery due to personal reasons, early NSCLC can be treated with stereotactic body radiotherapy (SBRT) or image-guided ablation (33). GGOs with solid component or growing GGO likely representing pre-malignant changes or early malignancies can also be amenable to non-surgical treatments. Image-guided techniques rely on thermal or electrical methods to directly destroy tumour cells, and are mostly done under CT guidance. The hybrid OR when combined with ENB biopsy allows for diagnosis of pre-malignant lesions or early stage NSCLC with frozen section or rapid on-site evaluation (ROSE), and same session treatment with either radiofrequency or microwave ablation (34).
Radiofrequency is the most widely used ablative technique for the treatment of lung cancers. Most clinically available devices function in the 375–500 kHz range. The electrode is placed into the lesion under image guidance, then coupled to a radiofrequency generator, which produces a voltage between the electrode and the grounding pad, causing an oscillating electric field. Electrons collide with adjacent molecules under the field inducing frictional heating, and immediate cell death is achieved when temperature surpass 60 degrees Celsius (35). To date, the safety, local control rates and survival rates have been published in non-randomized institutional series (36,37) and one multi-center trial (38), but all involving percutaneous placement of the electrode. Correct placement of the radiofrequency electrode into the target tumour was feasible in 99% of patients, survival rate was at 92% at 1 year and 73% at 2 years in patients with NSCLC (35), and smaller tumours particularly those less than 3cm are associated with better local control rates.
Recently, a more popular energy source for ablation has been microwave, due to its ability to create larger and more predictable ablation zones compared with RF. Similar to RF, a needle-like antenna is placed into target lesion under CT guidance to directly deliver microwave energy into a tumour. The clinically available microwave applicators usually operate in the 900–2,450 MHz range (39). Microwave induces kinetic energy in the highly polar water molecules, causing them to spin rapidly, causing cell death by heat. Tumour necrosis with central air cavities on post-ablative CT is indicative of treatment success and associated with a lower cancer-specific mortality (40). Recurrence rate was approximately 17% at 13 months for tumours under 3 cm and 31% for those larger than 3 cm (41).
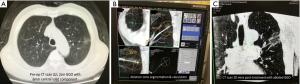
We recently performed Asia-Pacific’s first non-invasive bronchoscopic microwave ablation (BMA) in the HOR setting in March 2019. The lung lesions were localized and biopsied with ENB, followed by placement of flexible microwave ablation catheter through the tumour via EWC and ablation performed in a single session in the HOR. The target lesion should be smaller than 3 cm because of the limited treatment zone size of the microwave catheter, and the tumour should be located at the outer two-thirds of the lung and away from major blood vessels (Figure 5).
Comments: the future of hybrid operating room
Each of the four essential aspects of the HOR are undergoing rapid development and upgrade. The resolution of CT and fluoroscopy, the imaging software, and the accuracy of endobronchial navigation platforms will continue to improve. Our experience of other bronchoscopic navigation systems that does not or are less dependent on electromagnetic navigation such as the virtual bronchoscopic navigation (VBN) system LungPoint® (Bronchus Medical, Inc., San Jose, CA, USA), and more fluoroscopic based navigation LungVisionTM (Body Vision Medical Ltd, Israel) are equally capable of working within the hybrid operating room environment, and can boast navigation accuracies of several millimetres. Future robotic navigational bronchoscopy systems will likely takeover some of the burden of clinician-directed bronchoscopies with better spacial orientation and improved accuracy, and thus may decrease dependence on imaging. Bronchoscopic ablative techniques have recently spread across the globe with promising safety and efficacy. Various novel combinations of the four aspects of a successful hybrid operating room also give rise to ample research opportunities. With increasing emphasis on patient-centred care, a one-stop solution for early lung cancers will likely gain popularity and favour (42).
Conclusions
Real-time imaging from HOR improves positional accuracy of navigation and marking tools for biopsy and localization, and the one-stop centralization of marker placement and immediate treatment can drastically reduce complications like marker dislodgement, dye diffusion and pneumothorax. Combining the current navigation platforms with CBCT and various endobronchial treatment options, for instance ENB-guided microwave ablation, allow the potential for precise personalized non-invasive localized therapy. The hybrid operating room for thoracic surgery in selected patients is a cost-effective streamlined solution for the diagnosis and treatment of early stage lung cancers.
Acknowledgments
None.
Footnote
Conflicts of Interest: Calvin S. H. Ng is a consultant for Johnson and Johnson and Medtronic, USA. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg 2014;46:1-7. [Crossref] [PubMed]
- Ng CS, Lau KK, Gonzalez-Rivas D, et al. Evolution in surgical approach and techniques for lung cancer. Thorax 2013;68:681. [Crossref] [PubMed]
- Tempany CM, Jayender J, Kapur T, et al. Multimodal imaging for improved diagnosis and treatment of cancers. Cancer 2015;121:817-27. [Crossref] [PubMed]
- Zhao ZR NS. Image-Guided Uniportal VATS in the Hybrid Operating Room. In: Gonzalez-Rivas D. NC, Rocco G., D’Amico T. (eds), editor. Atlas of Uniportal Video Assisted Thoracic Surgery. Singapore: Springer; 2019:269-78.
- Schafer S, Nithiananthan S, Mirota DJ, et al. Mobile C-arm cone-beam CT for guidance of spine surgery: image quality, radiation dose, and integration with interventional guidance. Med Phys 2011;38:4563-74. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Yu PS, et al. Image-guided localization of small lung nodules in video-assisted thoracic surgery. J Thorac Dis 2016;8:S731-7. [Crossref] [PubMed]
- Schroeder C, Chung JM, Mitchell AB, et al. Using the Hybrid Operating Room in Thoracic Surgery: A Paradigm Shift. Innovations (Phila) 2018;13:372-7. [Crossref] [PubMed]
- Ng CS, Zhao ZR, Lau RW. Tailored Therapy for Stage I Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:268-70. [Crossref] [PubMed]
- Yu PSY, Man Chu C, Lau RWH, et al. Video-assisted thoracic surgery for tiny pulmonary nodules with real-time image guidance in the hybrid theatre: the initial experience. J Thorac Dis 2018;10:2933-9. [Crossref] [PubMed]
- Kidane B, Yasufuku K. Advances in Image-Guided Thoracic Surgery. Thorac Surg Clin 2016;26:129-38. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Ng CS. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S319-27. [PubMed]
- Zhao ZR. NS. Hookwire Localization of Pulmonary Nodules in Uniportal VATS. In: Gonzalez-Rivas D. NC, Rocco G., D’Amico T. (eds), editor. Atlas of Uniportal Video Assisted Thoracic Surgery. Singapore: Springer; 2019. p. 95-100.
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Obeso A, Ng CSH. Electromagnetic navigation bronchoscopy in the thoracic hybrid operating room: a powerful tool for a new era. J Thorac Dis 2018;10:S764-8. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800-5. [Crossref] [PubMed]
- Ng CS, Yu SC, Lau RW, et al. Hybrid DynaCT-guided electromagnetic navigational bronchoscopic biopsydagger. Eur J Cardiothorac Surg 2016;49 Suppl 1:i87-8. [PubMed]
- Ng CSH, Zhao Z, Long H, et al. Electromagnetic Navigation Bronchoscopy Triple Contrast Dye Marking for Lung Nodule Localization. Thorac Cardiovasc Surg 2019. [Epub ahead of print]. [PubMed]
- Ng CS, Wong RH, Lau RW, et al. Single port video-assisted thoracic surgery: advancing scope technology. Eur J Cardiothorac Surg 2015;47:751. [Crossref] [PubMed]
- Ng CS, Pickens A, Siegel JM, et al. A novel narrow profile articulating powered vascular stapler provides superior access and haemostasis equivalent to conventional devicesdagger. Eur J Cardiothorac Surg 2016;49 Suppl 1:i73-8. [PubMed]
- Ng CS, Kim HK, Wong RH, et al. Single-Port Video-Assisted Thoracoscopic Major Lung Resections: Experience with 150 Consecutive Cases. Thorac Cardiovasc Surg 2016;64:348-53. [Crossref] [PubMed]
- Ng CSH, MacDonald JK, Gilbert S, et al. Expert Consensus Statement on Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer. Innovations (Phila) 2019;14:87-9. [Crossref] [PubMed]
- Ng CSH, MacDonald JK, Gilbert S, et al. Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systemic Review and Meta-Analysis. Innovations (Phila) 2019;14:90-116. [Crossref] [PubMed]
- Ng CS, Wong RH, Lau RW, et al. Minimizing chest wall trauma in single-port video-assisted thoracic surgery. J Thorac Cardiovasc Surg 2014;147:1095-6. [Crossref] [PubMed]
- Ng CSH, Man Chu C, Kwok MWT, et al. Hybrid DynaCT scan-guided localization single-port lobectomy. Chest 2015;147:e76-8. [Crossref] [PubMed]
- Zhao ZR, Lau RWH, Ng CSH. Hybrid Theater and Uniportal Video-Assisted Thoracic Surgery: The Perfect Match for Lung Nodule Localization. Thorac Surg Clin 2017;27:347-55. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer</= 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Zhao ZR NC. Awake, non-intubated transpleural surgery. In: Joseph LoCicero RF, Yolonda Colson, Gaetano Rocco (eds), editor. Shield’s General Thoracic Surgery. 8 ed.: Wolters Kluwer; 2018:497-501.
- Zhao ZR, Lau RW, Ng CS. Non-intubated video-assisted thoracic surgery: the final frontier? Eur J Cardiothorac Surg 2016;50:925-6. [Crossref] [PubMed]
- Tacconi F, Pompeo E. Non-intubated video-assisted thoracic surgery: where does evidence stand? J Thorac Dis 2016;8:S364-75. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Ng C. Advances in Uniportal Video-Assisted Thoracoscopic Surgery: Pushing the Envelope. Thorac Surg Clin 2016;26:187-201. [Crossref] [PubMed]
- Zhao ZR, Lau RWH, Ng CSH. Catheter-based alternative treatment for early-stage lung cancer with a high-risk for morbidity. J Thorac Dis 2018;10:S1864-70. [Crossref] [PubMed]
- Zhao ZR, Lau RWH, Long H, et al. Novel method for rapid identification of micropapillary or solid components in early-stage lung adenocarcinoma. J Thorac Cardiovasc Surg 2018;156:2310-8.e2. [Crossref] [PubMed]
- Organ LW. Electrophysiologic principles of radiofrequency lesion making. Appl Neurophysiol 1976;39:69-76. [PubMed]
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 2007;243:268-75. [Crossref] [PubMed]
- Ambrogi MC, Fanucchi O, Cioni R, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thorac Oncol 2011;6:2044-51. [Crossref] [PubMed]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621-8. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25 Suppl 1:S69-83. [Crossref] [PubMed]
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 2008;247:871-9. [Crossref] [PubMed]
- Healey TT, March BT, Baird G, et al. Microwave Ablation for Lung Neoplasms: A Retrospective Analysis of Long-Term Results. J Vasc Interv Radiol 2017;28:206-11. [Crossref] [PubMed]
- Ng CSH, He JX, Rocco G. Innovations and technologies in thoracic surgery. Eur J Cardiothorac Surg 2017;52:203-5. [Crossref] [PubMed]


