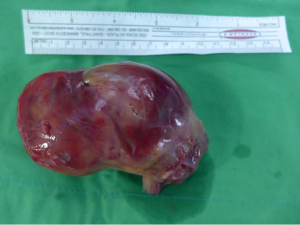
Right ventricle inflow obstructing mass proven to be a synovial sarcoma
Introduction
Approximately one quarter of all cardiac tumors exhibit some features of malignancy. The majority of malignant primary cardiac tumors are sarcomas, usually angiosarcomas or rhabdomyosarcomas (1). Synovial sarcoma (SS) is a clinically and histomorphologically well-defined soft tissue tumor that is extremely uncommon in joint cavities and has no apparent relation to synovial structures. SSs have been described at other unusual sites including the heart, pleura, kidney, prostate, liver, mediastinum, gastrointestinal tract, and peripheral nerve (2-7). A SS arising primarily from heart is uncommon and is usually identified at a late stage because of its nonspecific presentation and difficulty in obtaining a biopsy from this site. Complete surgical resection combined with chemoradiotherapy continues to remain the fundamental strategy for sarcoma management. This case describes our experiences with the presentation, diagnosis, and management of a patient with SS of the heart.
Case
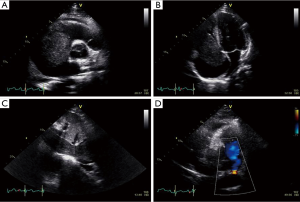
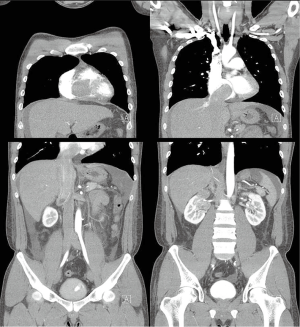
A 27-year-old man presented with a 1-month history of dyspnea on exertion, nausea, general weakness, and palpitations. He had no remarkable medical or family history. Vital signs at the time of the first admission revealed temperature, 36.5 °C; respiration, 27/min; pulse, 110/min; and blood pressure, 120/80 mmHg. An examination showed a faint continuous murmur with clear breathing sounds and mild peripheral pitting edema on both legs. Initial laboratory findings showed elevated levels of serum bilirubin, 2.2 mg/dL; aspartate aminotransferase, 194 IU/L; alanine aminotransferase, 343 IU/L; creatinine, 1.49 mg/dL; and pro-brain natriuretic peptide, 3267.2 pg/mL with prolonged prothrombin time. A chest X-ray showed cardiomegaly and pleural effusion. Transthoracic echocardiography revealed a large mass in the right heart that nearly obstructed the right ventricle inflow tract during systole and a small 0.4 cm sized patent ductus arteriosus (Figure 1). Subsequent computed tomography showed a heterogenous enhancing tumor like lesion along the right ventricle, right atrium, inferior vena cava (IVC), right renal vein, and upper pole of the right kidney (Figure 2).
The decision was made to resect the mass for a definite diagnosis and to restore the right heart failure with congestive hepatopathy and nephropathy. Aggressive surgical management was done with radical nephrectomy, IVC mass excision, and intra-arterial tumor resection (Figure 3). The cardiac tumor was about 10.5 cm × 5.5 cm × 5.0 cm sized and connected to IVC mass without adhesion. There were no adhesion of the tumor at all and no lymph node enlargement, intra-atrial tumor was cut and removed from the near IVC and hepatic vein. From abdomen incision, after the incision of orifice of right renal vein that extend to the IVC, nephrectomy was done. The remained IVC tumor was removed totally.
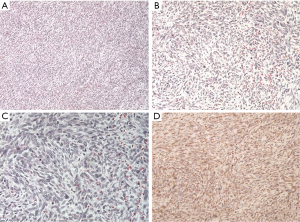
Histological features revealed a monophasic SS, composed of fairly low uniform grade spindle cells. Immunohistochemical staining was positive for vimentin, C-kit, Bcl-2, and negative for cytokeratin (CK), smooth muscle actin, CD34, desmin, S100, CK7, and CK19. Transducin-like enhancer of split TLE1 was positive with the characteristic t(X; 18) (p11.2; q11.2) translocation, the SYT-SSX fusion oncogene was not present (Figure 4).
The postoperative period was uneventful. He was discharged on postoperative day 10 by arrangement with an oncologist. He underwent four cycles of chemotherapy with 40 mg/m2 of adriamycine and 5,000 mg/m2 of ifosfamide. To date, he is alive with no significant clinical issues 5 months after primary surgery.
Discussion
Sarcomas are common between the third and fifth decades of life and the right atrium is the most frequently affected (1). They have a wide variety of morphologies due to their mesenchymal origin. The SS in this case was located from the right ventricle to the right kidney. Except the kidney and adjacent large vessels, no definite adhesions or extensions were found. However the exact origin of this patient’s SS is still uncertain because of the side of uncertified microvascular invasion and the possibility of a widely distributed origin. SS is typically an aggressive tumor, and survival is usually less than nine months, even with surgery and adjuvant chemoradiotherapy. As with all malignant tumors of the heart, the characteristics of a SS can be life-threatening because of obstructed intracardiac blood flow, interference with valve function, and rhythm disturbances, as well as pericardial tamponade resulting from local invasion (8). Because cardiac SSs are rare, prognostic factors are hard to ascertain, but younger age at diagnosis, absence of complex chromosomal abnormalities, and origin of the tumor from the pericardium seem to be favorable factors (9).
SSs have biphasic and monophasic histological patterns. The classic biphasic SS has mixed epithelial and spindle cell components, whereas the monophasic SS has only the spindle cell component. In most cases of biphasic SSs, the expression of both CK and vimentin is seen, and almost always at least one epithelial cell marker is expressed, although expression may be focal. In monophasic SS, tumor cells stain diffusely positive for vimentin and variably positive for epithelial proteins. The hallmark of the SS diagnosis is detection of the translocated chromosome t(X; 18) (p11.2;q11.2), the SYT-SSX fusion oncogene, which is present in more than 90% of the SSs (10). TLE1 is one of four TLE genes that encode human transcriptional repressors homologous to the Drosophila corepressor groucho (11). TLE1 is a sensitive and specific immunohistochemical marker for SS, performing better than other known immunohistochemical markers, and can significantly aid in the pathologic diagnosis (12). We confirmed the diagnosis in this case using ancillary diagnostic methods such as cytogenetics and fluorescence in situ hybridization and validated that TLE1 was positive even though the SYT-SSX fusion oncogene was negative.
Treatment for SS is a combination of surgical resection, radiotherapy, and chemotherapy. Radical resection is the mainstay of treatment, although complete resection is seldom possible. Adjuvant radiotherapy is usually recommended in cases of extensive resection of a large tumor or incomplete resection. Adjuvant chemotherapy is needed but no standard medical therapy exists due to the rarity of SS. Therefore, more aggressive and complete surgical resection allows the best chance for palliation and survival due to SS.
We herein reported a case of cardiac SS immunohistochemically confirmed by TLE1 treated with surgical resection and adjuvant chemotherapy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Shapiro LM. Cardiac tumours: diagnosis and management. Heart 2001;85:218-22. [PubMed]
- Wang JG, Li NN. Primary cardiac synovial sarcoma. Ann Thorac Surg 2013;95:2202-9. [PubMed]
- Tuncer ON, Erbasan O, Golbasi I. Primary intravascular synovial sarcoma: case report. Heart Surg Forum 2012;15:E297-9. [PubMed]
- Sheu CC, Lin SF, Chiu CC, et al. Left atrial sarcoma mimicking obstructive pulmonary disease. J Clin Oncol 2007;25:1277-9. [PubMed]
- Kang MK, Cho KH, Lee YH, et al. Primary synovial sarcoma of the parietal pleura: a case report. Korean J Thorac Cardiovasc Surg 2013;46:159-61. [PubMed]
- Hussain S, Khan AA, Khan Q. Synovial sarcoma of the heart. J Coll Physicians Surg Pak 2012;22:723-5. [PubMed]
- de Zwaan C, Bekkers SC, van Garsse LA, et al. Primary monophasic mediastinal, cardiac and pericardial synovial sarcoma: a young man in distress. Neth Heart J 2007;15:226-8. [PubMed]
- Alsara O, Rayamajhi S, Ghanem F, et al. Primary cardiac pleomorphic sarcoma presenting as back pain in an 18-year-old man. Tex Heart Inst J 2013;40:339-42. [PubMed]
- Varma T, Adegboyega P. Primary cardiac synovial sarcoma. Arch Pathol Lab Med 2012;136:454-8. [PubMed]
- Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors. Synovial sarcoma. Cancer Genet Cytogenet 2002;133:1-23. [PubMed]
- Stifani S, Blaumueller CM, Redhead NJ, et al. Human homologs of a Drosophila Enhancer of split gene product define a novel family of nuclear proteins. Nat Genet 1992;2:119-27. [PubMed]
- Terry J, Saito T, Subramanian S, et al. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol 2007;31:240-6. [PubMed]