
Intensity-modulated radiotherapy does not decrease the risk of malnutrition in esophageal cancer patients during radiotherapy compared to three-dimensional conformal radiation therapy
Introduction
Esophageal cancer is a serious malignancy with regards to mortality and prognosis. Squamous cell carcinoma is the most common histological type of esophageal cancer worldwide, with a higher incidence in developing nations (1). Although treatment technologies have improved in recent years, the five-year survival rate remains low (ranging from 15% to 25%) (2). Radiotherapy plays a crucial role in the management of esophageal cancer (3,4). Three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT) are the most common technologies used for esophageal cancer treatment. 3D-CRT realizes the distribution of radiation fields in polyhedral and non-concentric areas, which contributes to better target coverage and decreased toxicity to normal organs compared to 2D-CRT. IMRT has become increasingly popular for the treatment of esophageal and other cancers (5). The superiority of the dosimetry distribution of IMRT leads to superiority in clinical efficacy. It has been reported to significantly reduce the dosage to the lung (5) and heart (6) in esophageal cancer radiotherapy. Furthermore, IMRT is associated with a lower risk of grade 3 or higher non-hematologic toxicity (7) and a higher five-year survival rate than 3D-CRT (8,9).
Nutrition status is of great importance for tumor patients’ survival. Malnutrition frequently occurs in cancer patients and has negative effects on clinical outcomes (10). A moderate degree of malnutrition and a severe degree of malnutrition were observed in 76% and 12% of cancer patients, respectively (11). The prevalence of malnutrition in esophageal cancer patients has been reported to be about 60.2% (12). However, only 28.4% of non-malnourished patients and 57.6% of malnourished patients received nutrition support (12).
Radiotherapy increases the risk of malnutrition. One study showed that 74% of patients were malnourished at the end of radiotherapy while this proportion was only 10% before radiotherapy (13). The increase of malnutrition during radiotherapy was also reported in breast, lung, stomach, and colorectal cancer patients (14). Brown et al. (15) compared the difference of 3D-CRT and helical-IMRT in body weight change and the need for tube feeding. It was found that both groups had a median weight loss of 45%, a high incidence of tube feeding, and severe weight loss. Nutrition intervention remains critical despite advances in radiotherapy techniques. However, no research has been reported in esophageal cancer patients. In this study, we aimed to compare the effect of IMRT and 3D-CRT on the nutritional status of esophageal cancer patients.
Methods
Subjects
We retrospectively included 95 esophageal cancer patients receiving radiotherapy between January 2018 and August 2018 in the Qilu Hospital of Shandong University. All patients received standardized nutritional management. Nutrition Risk Screening 2002 (NRS-2002) screening was performed on a regular basis, and patients with nutritional risk were given a PG-SGA score. For patients at risk, we consulted the nutrition department and gave nutritional intervention. After nutrition screening and examination, a five-step nutritional treatment was given according to the specific nutritional risk of patients, including modification of basic nutrition, oral nutritional supplements (ONS), enteral nutrition (EN), and parenteral nutrition (PN). The most common nutritional approach is the modification of regular nutritional intake due to dysphagia. If the intake of food with a normal diet is insufficient despite these adjustments, we opt for an additional nutritional intake using ONS. EN is the least invasive form of artificial nutritional therapy. Nutrient solutions are delivered through a tube or a stoma. When a patient being treated for esophageal cancer experiences a partial or complete gastrointestinal failure and sufficient nutritional and energy intake cannot be administered by means of EN, we opt for a supplementary or complete PN (16). Patients included who had malnutrition received individualized dietary counseling and ONS and no patients received EN or PN. Nutrition Risk Screening 2002 (NRS-2002), body weight, blood routine, and liver function are examined every week during radiotherapy to assess the patient’s metabolic state, response to nutritional therapy, and utilization of nutrients. For comparison, we included the NRS-2002 score, body weight, body mass index (BMI), hemoglobin (Hb), lymphocyte, pre-albumin, total protein, albumin, and globulin at the beginning, the second week, and the end of radiotherapy from the medical records. Prevalence of radiation esophagitis and treatment completion were also included for analysis. The esophagitis grade standard, as defined by the Acute Toxicity Grading System of The Radiation Therapy Oncology Group (RTOG), was used. We divided esophagitis grades 0 to 1 into one group, and radioactive esophagitis of grade 2 or greater into the other group.
The same dosimetric constraints were used for IMRT and 3D-CRT. The main dosimetric constraints were as follows: the prescribed dose contains 95% of the target area, the V20 of the lung is less than 30%; the V30 is less than 20%, and the other organs at risk (OARs) are in accordance with RTOG’s 0615 protocols [the dose-volume histogram parameter of Vx was defined as the percentage of total organ volume receiving a radiation dose of x (Gy) or more]. For radical radiotherapy, gross tumor volume (GTV) includes imaging and gastroscopy of visible primary tumors and any positive lymph nodes. Clinical tumor volume (CTV) consists of a longitudinal area 3–5 cm away from esophageal tumors, 0.8 cm outside the esophagus and any positive lymph nodes, and area delineation. For postoperative adjuvant radiotherapy for esophageal cancer, CTV consists of the area of the tumor bed, positive lymph nodes, and area delineation.
The staging standard we adopted (including pathological staging and clinical staging) is the 8th edition of the TNM staging criteria for esophageal cancer co-published by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) (17,18). Neoadjuvant radiotherapy patients were not involved in our study. We included definitive and adjuvant radiotherapy patients. For patients who could not receive surgery or refused surgery, definitive radiotherapy was adopted. Adjuvant radiotherapy was used for patients receiving surgery. In regards to chemotherapy methods, monotherapy, doublet therapy, and triple therapy were adopted. Docetaxel or Tegio capsules were used for monotherapy. The combination of docetaxel and platinum was used for doublet therapy. The triple therapy was a combination of docetaxel, Tegio, and irinotecan. We defined concurrent chemoradiotherapy as the completion of 2 cycles of chemotherapy during radiotherapy. All concurrent chemoradiotherapy patients in our study completed 2 cycles of chemotherapy during radiotherapy. Patients with missing data or receiving radiotherapy not only for esophageal cancer control were excluded. None of the patients received a blood transfusion.
This study was approved by the Ethics Committee of Qilu Hospital. All participants were informed about the purpose, procedures, benefits, and potential risks of the study and their written consents were obtained.
Statistical analysis
Paired-T test was used to evaluate the nutrition status during radiotherapy in each group. Chi-square test and independent-sample T-test were applied to compare the nutrition status changes between the IMRT and 3D-CRT groups. P values <0.05 were considered statistically significant. All tests were two-sided. Statistical analyses were performed by using SPSS software (version 19.00; SPSS Inc., Chicago, IL, USA).
Results
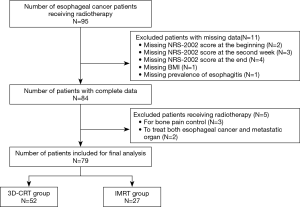
Ninety-five esophageal cancer patients receiving radiotherapy were first included. Eleven patients with missing data were excluded, while 3 patients receiving radiotherapy for bone pain control, and 2 for esophageal cancer along with metastatic organ treatment were excluded. Finally, 79 patients (52 in the 3D-CRT group and 27 the in IMRT group) were included in this study (Figure 1).
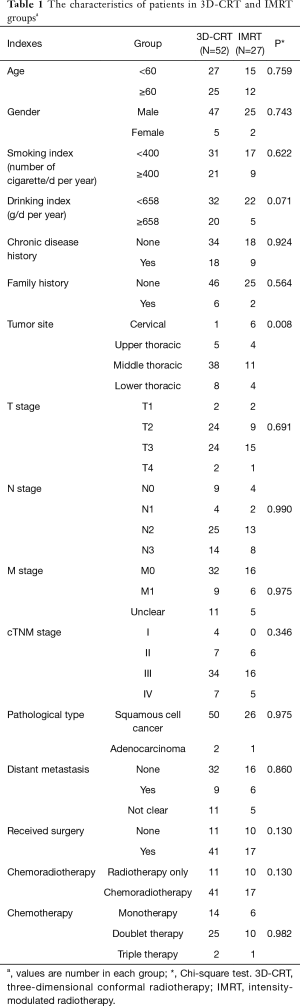
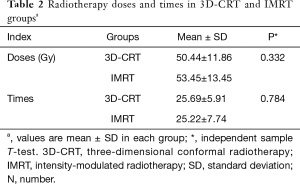
Firstly, we compared the baseline characteristics of the two groups. There was no difference in gender, age, smoking, drinking, chronic disease history, family history, TNM stage, distant metastasis, pathological type, and treatments. However, the difference was significant in the tumor site. Patients with cervical esophageal cancer in the IMRT group were more in number than those in the 3D-CRT group (P=0.008) (Table 1). The radiotherapy doses in the IMRT and 3D-CRT groups were 50.44±11.86 and 53.45±13.45 Gy (P=0.332), respectively, and the total radiotherapy times were 25.69±5.91 and 25.22±7.74 (P=0.784), respectively (Table 2).
Full table
Full table
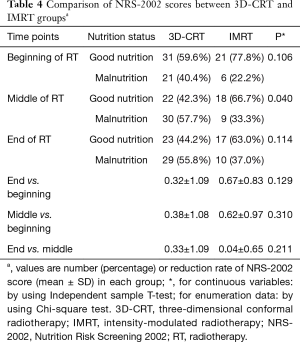
NRS-2002 score
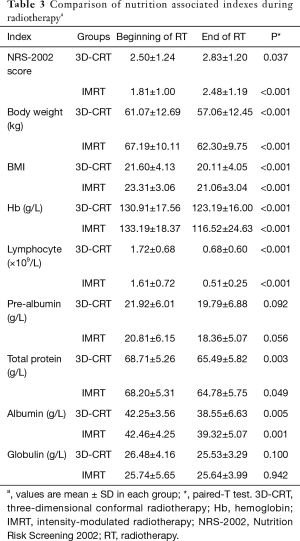
According to NRS-2002 nutritional screening criteria, patients with an NRS-2002 score ≥3 were regarded as having malnutrition. Paired-T test indicated that the NRS-2002 score increased during radiotherapy treatment in both the 3D-CRT and IMRT groups (P=0.037 and P<0.001) (Table 3). Furthermore, we found that the malnutrition status did not differ in both groups at the baseline (40.4% vs. 22.2%, P=0.106). In the second week, 3D-CRT generated higher malnutrition probability than IMRT (57.7% vs. 33.3%, P=0.040). At the end of radiotherapy, there was no statistically significant difference between the two groups (55.8% vs. 37.0%, P=0.114). We further compared the changes in NRS-2002 scores during radiotherapy between the two groups. However, no significant statistical differences were found (all P>0.05) (Table 4).
Full table
Full table
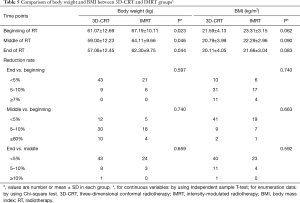
Body weight
Body weight decreased at the end of radiotherapy compared with the beginning in both the 3D-CRT and IMRT groups (both P<0.001) (Table 3). The baseline of body weight in the IMRT group was higher than that in the 3D-CRT group (67.19±10.11 vs. 61.07±12.69, P=0.023), which might have led to the difference between the two groups in the second week (64.11±9.66 vs. 59.00±12.23, P=0.046) and the end of radiotherapy (62.30±9.75 vs. 57.06±12.45, P=0.044). To compare the dynamic changes of weight during radiotherapy, we divided each group into three subgroups according to the boundaries of 5% and 10% between any two-time points (the end vs. the beginning, the second week vs. the beginning, and the end vs. the second week). However, no significant statistical differences were found (all P>0.05) (Table 5).
Full table
BMI
BMI index was compared between the two groups. Firstly, we found that BMI was reduced at the end of radiotherapy than at the beginning (both P<0.001) (Table 3). Then, we compared the IMRT and 3D-CRT groups. We found that there were no significant differences in BMI between the two groups at the beginning, middle, and end of radiotherapy. For the dynamic change of BMI during radiotherapy, we looked for the difference between any two time points. No statistical differences between the two groups were found (all P>0.05) (Table 5).
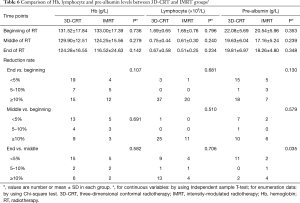
Hb
Hb level was also significantly reduced during radiotherapy in both groups (both P<0.001) (Table 3). We also analyzed its difference between the two groups, and found that the differences in Hb levels between the two groups at the beginning, middle, and end of radiotherapy were not significant, and there were also no statistical differences for the dynamic changes of Hb during the process of radiotherapy between the two groups (all P>0.05) (Table 6).
Full table
Lymphocyte count
Lymphocyte count was decreased during radiotherapy in both groups (both P<0.001) (Table 3). No difference was found for lymphocyte count at each point of radiotherapy between the two groups. Moreover, there were no statistical differences when comparing the dynamic changes during radiotherapy (all P>0.05) (Table 6).
Pre-albumin
No significant decrease was found during radiotherapy in both groups (P=0.092 in the 3D-CRT group and P=0.056 in the IMRT group) (Table 3). Moreover, we found that there were no significant differences when comparing pre-albumin level at the beginning, middle, or endpoints during radiotherapy nor were there any dynamic changes during radiotherapy between the 3D-CRT and IMRT groups (all P>0.05) (Table 6).
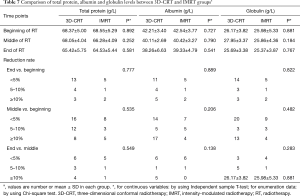
Total protein
Total protein level was significantly reduced during radiotherapy in both the 3D-CRT and IMRT groups (P=0.003 and 0.049, respectively) (Table 3). No differences were found when comparing its levels at the 3-time points, and no differences were found in dynamic changes during radiotherapy between 3D-CRT and IMRT (all P>0.05) (Table 7).
Full table
Albumin
Albumin levels were significantly reduced during radiotherapy in both the 3D-CRT and IMRT groups (P=0.005 and 0.001, respectively) (Table 3). No differences were found when comparing its levels at the beginning, middle or endpoints, nor were there any dynamic changes during radiotherapy between the IMRT and 3D-CRT groups (all P>0.05) (Table 7).
Globulin
No significant decrease was found during radiotherapy in both groups (both P>0.05) (Table 3). Furthermore, no differences were found when comparing its level at the 3 time points, nor were there any dynamic changes during radiotherapy (all P>0.05) (Table 7).
Radiation esophagitis
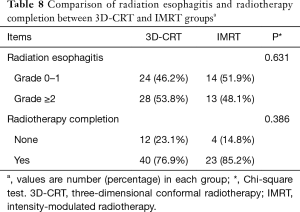
We compared the prevalence of radiation esophagitis at the end of radiotherapy. In all, 28 of 52 (53.8%) patients were diagnosed with radiation esophagitis in the 3D-CRT group, while 13 of 27 (48.1%) patients were diagnosed in the IMRT group. No difference between 3D-CRT and IMRT was found (P=0.631) (Table 8).
Full table
Treatment completion
The treatment completion rate was analyzed. In all, 40 of 52 (76.9%) patients completed the radiotherapy plan in the 3D-CRT group, while 23 of 27 (85.2%) patients finished the treatment plan in the IMRT group. There was no significant difference between the two groups (P=0.386) (Table 8).
Discussion
Radiation therapy is a double-edged sword, but the relevant technological innovations can reduce the side effects of normal organs and improve treatment efficacy. However, side effects will still inevitably occur over the course of treatment. It has been reported that more than 70% of esophageal cancer patients experience malnutrition, which is caused mainly by anorexia and dysphagia, before the treatment starts (19). Dysphagia-related nutrition deficiency may be even worse during the treatment, and, as a result, weight loss may occur. During RT, nutritional interventions positively influence outcomes, including improving nutritional intake and status, decreasing morbidity of RT toxicity, and improving quality of life (20). A systematic review showed the beneficial effects of individualized dietary counseling on nutritional status and quality of life, compared to no counseling or standard nutritional advice (21). It was also reported that after preforming enteral tube feeding with the energy of 25 kcal × kg/d in esophageal cancer patients during radiotherapy, prognostic nutritional index and the key nutritional index reflecting prognosis, significantly decreased (22). Cong et al. (23) found that nutritional status was significantly improved in a nutrition support group. Meanwhile, nutritional interventions improved the completion of radiotherapy and decreased the average length of hospital stay of esophageal cancer patients. The major goal of nutrition intervention is to favorably influence body composition, with the potential to improve cancer therapy outcomes, morbidities, and ultimately, prognosis. To be effective, individualized counselling has to be based on a thorough assessment of various nutritional and clinical parameters: nutritional status and dietary intake, usual dietary pattern, intolerances or food aversions, patients’ psychological status, autonomy, cooperation, and need for help or support of others in the act of eating (24). A five-step nutritional treatment was applied to malnutrition patients. In our department, we carry out nutrition intervention for all malnutrition patients. Therefore, we did not specifically analyze the role of nutrition intervention.
IMRT technology achieves better target dosimetry distribution compared to 3D-CRT and improves clinical outcomes. It improves the local control rate and long term survival while reducing side effects in various cancers like nasopharyngeal carcinoma (25,26), head and neck cancer (27), and pancreatic cancer (28). We investigated whether IMRT generates advantages in nutrition status. However, this study indicates that IMRT technology did not decrease malnutrition risk compared to 3D-CRT.
The esophagus is a string organ. The radiation field covers cancerous tissues and part of the normal esophageal tissues. The radiation dose is relevant to radiation esophagitis, which is the main cause of eating pain and further malnutrition. It always appears at about 2–3 weeks from the beginning of radiotherapy and lasts for more than 2 weeks after radiotherapy (29). In our study, the incidence of radiation esophagitis between the two groups was not significant, which may be the main reason for the unobvious difference in nutritional status between the two groups. Advances in IMRT technology manifest in the reduction in dosimetry distribution on surrounding normal tissues, but not the effect on nutritional status during radiotherapy. A similar conclusion has been reported in head and neck cancer (15), as mentioned previously.
The proportion of cervical esophageal cancer patients was higher in the IMRT group than the 3D-CRT group. IMRT is recommended for cervical esophageal cancer treatment because normal radiosensitive structures in the head and neck are close to the radiation area, and higher radiation doses are commonly required in this area (3). In cervical esophageal cancer, IMRT provides superior target volume coverage and conformity, with decreased dose to normal structures (30). It was reported that higher RT doses were associated with a borderline improved OS (31). However, the number of patients in the 3D-CRT group was not enough for statistical analysis, so we did not compare the effect of the two technologies on cervical esophageal cancer.
There are some limitations to this study. Firstly, this study is retrospective, not prospective, and has a small sample size. Secondly, all patients come from the same hospital which may lead to selection bias. Thirdly, chemotherapy methods of concurrent chemoradiotherapy are not uniform, but we did not compare different chemotherapy methods because of the small sample size. Moreover, in this study, ONS were used for all patients with an NRS-2002 score less than 3. PN and EN treatments were not used in our study. Therefore, we did not analyze the effect of different nutrition intervention methods. A multi-center study with a large sample is required to validate these findings.
In conclusion, malnutrition occurs during radiotherapy, and IMRT did not significantly decrease the risk of this malnutrition in esophageal cancer patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Qilu Hospital. All participants were informed about the purpose, procedures, benefits and potential risks of the study, and their written consents were obtained.
References
- Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014;6:112-20. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Zhang M, Wu AJ. Radiation techniques for esophageal cancer. Chin Clin Oncol 2017;6:45. [Crossref] [PubMed]
- Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97-110. [Crossref] [PubMed]
- Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol 2005;77:247-53. [Crossref] [PubMed]
- Grosshans D, Boehling NS, Palmer M, et al. Improving cardiac dosimetry: Alternative beam arrangements for intensity modulated radiation therapy planning in patients with carcinoma of the distal esophagus. Pract Radiat Oncol 2012;2:41-5. [Crossref] [PubMed]
- Freilich J, Hoffe SE, Almhanna K, et al. Comparative outcomes for three-dimensional conformal versus intensity-modulated radiation therapy for esophageal cancer. Dis Esophagus 2015;28:352-7. [Crossref] [PubMed]
- Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs. intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078-85. [Crossref] [PubMed]
- Xu D, Li G, Li H, et al. Comparison of IMRT versus 3D-CRT in the treatment of esophagus cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7685. [Crossref] [PubMed]
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11-48. [Crossref] [PubMed]
- Álvaro Sanz E, Garrido Siles M, Rey Fernandez L, et al. Nutritional risk and malnutrition rates at diagnosis of cancer in patients treated in outpatient settings: Early intervention protocol. Nutrition 2019;57:148-53. [Crossref] [PubMed]
- Hébuterne X, Lemarie E, Michallet M, et al. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr 2014;38:196-204. [Crossref] [PubMed]
- Citak E, Tulek Z, Uzel O. Nutritional status in patients with head and neck cancer undergoing radiotherapy: a longitudinal study. Support Care Cancer 2019;27:239-47. [Crossref] [PubMed]
- Unsal D, Mentes B, Akmansu M, et al. Evaluation of nutritional status in cancer patients receiving radiotherapy: a prospective study. Am J Clin Oncol 2006;29:183-8. [Crossref] [PubMed]
- Brown T, Banks M, Hughes BG, et al. New radiotherapy techniques do not reduce the need for nutrition intervention in patients with head and neck cancer. Eur J Clin Nutr 2015;69:1119-24. [Crossref] [PubMed]
- Jordan T, Mastnak DM, Palamar N, et al. Nutritional Therapy for Patients with Esophageal Cancer. Nutr Cancer 2018;70:23-9. [Crossref] [PubMed]
- Rice TW, Gress DM, Patil DT, et al. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:304-17.
- Mo R, Chen C, Pan L, et al. Is the new distribution of early esophageal adenocarcinoma stages improving the prognostic prediction of the 8(th) edition of the TNM staging system for esophageal cancer? J Thorac Dis 2018;10:5192-8. [Crossref] [PubMed]
- Di Fiore F, Lecleire S, Pop D, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol 2007;102:2557-63. [Crossref] [PubMed]
- Ravasco P, Monteiro-Grillo I, Marques Vidal P, et al. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005;27:659-68. [Crossref] [PubMed]
- Langius JA, Zandbergen MC, Eerenstein SE, et al. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr 2013;32:671-8. [Crossref] [PubMed]
- Ye Y, Xu Y, Fu Q, et al. Enteral Nutrition Support Does Not Improve PNI in Radiotherapy Patients with Locally Advanced Esophageal Cancer. Nutr Cancer 2019;71:223-9. [Crossref] [PubMed]
- Cong MH, Li SL, Cheng GW, et al. An Interdisciplinary Nutrition Support Team Improves Clinical and Hospitalized Outcomes of Esophageal Cancer Patients with Concurrent Chemoradiotherapy. Chin Med J (Engl) 2015;128:3003-7. [Crossref] [PubMed]
- Ravasco P. Nutrition in Cancer Patients. J Clin Med 2019. [Crossref] [PubMed]
- Moon SH, Cho KH, Lee CG, et al. IMRT vs. 2D-radiotherapy or 3D-conformal radiotherapy of nasopharyngeal carcinoma: Survival outcome in a Korean multi-institutional retrospective study (KROG 11-06). Strahlenther Onkol 2016;192:377-85. [Crossref] [PubMed]
- Zhang B, Mo Z, Du W, et al. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral Oncol 2015;51:1041-6. [Crossref] [PubMed]
- Gupta T, Agarwal J, Jain S, et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol 2012;104:343-8. [Crossref] [PubMed]
- Prasad S, Cambridge L, Huguet F, et al. Intensity modulated radiation therapy reduces gastrointestinal toxicity in locally advanced pancreas cancer. Pract Radiat Oncol 2016;6:78-85. [Crossref] [PubMed]
- Simone CB 2nd. Thoracic Radiation Normal Tissue Injury. Semin Radiat Oncol 2017;27:370-7. [Crossref] [PubMed]
- Fenkell L, Kaminsky I, Breen S, et al. Dosimetric comparison of IMRT vs. 3D conformal radiotherapy in the treatment of cancer of the cervical esophagus. Radiother Oncol 2008;89:287-91. [Crossref] [PubMed]
- McDowell LJ, Huang SH, Xu W, et al. Effect of Intensity Modulated Radiation Therapy With Concurrent Chemotherapy on Survival for Patients With Cervical Esophageal Carcinoma. Int J Radiat Oncol Biol Phys 2017;98:186-95. [Crossref] [PubMed]


