
Serum lactate dehydrogenase activity and its isoenzyme patterns in patients with pectus excavatum
Introduction
Pectus excavatum is the most common congenital disease of the chest wall, characterized as depression of the anterior chest wall producing a funnel-shaped chest (1,2). There have been many studies on cardiopulmonary compromise and operative techniques in pectus excavatum (3-5). Patients with pectus excavatum, except cases with severe compression of the internal organs such as the heart and inferior vena cava, are usually healthy, asymptomatic, and are not known to show any specific laboratory findings such that further testing is considered unnecessary (1,6). However, in our previous study on routine preoperative laboratory checkups, we found an elevation in serum total lactate dehydrogenase (LDH) before correction of the deformity and a decrease of total LDH after correction of the deformity (6). We hypothesized that these findings were caused by a combination of the disease entity and compression of the internal organs (6). However, the causes of these findings were not evaluated, because tests on LDH isoenzymes were not included in routine laboratory checkups. LDH is an enzyme found in almost all viable cells and plays an essential role in cell energy production (7). LDH is a tetrameric enzyme, composed of four subunits which are the M and H proteins (7,8). These two proteins comprise five possible isoenzymes: 4H (LDH1), 3H1M (LDH2), 2H2M (LDH3), 1H3M (LDH4), and 4M (LDH5) (7,8). These tetrameric isoenzymes show different tissue distributions (8). LDH1 (heart type) exists in the heart and in red blood cells, LDH2 exists in the reticuloendothelial system, LDH3 exists in the lungs, LDH4 exists in the kidneys, placenta, and pancreas, and LDH5 (muscle type) exists in the liver and striated muscle (8). LDH level is elevated due to various causes (7,8). Total LDH and its isoenzymes are usually examined to measure severity of tissue damage, to help identify the location, to monitor progression of severe infections or certain conditions, and to help screen some malignancies (7,9,10). In this study, we analyzed total LDH activity and its isoenzyme patterns to investigate the causes of these laboratory findings in patients with pectus excavatum.
Methods
From March 2014 to December 2018, patients with pectus excavatum who had undergone the Nuss procedure (NP) and bar removal (BR) and met the inclusion criteria were included. Inclusion criteria for this study were as follows: (I) no other diagnosed disease except pectus excavatum, (II) no drug or medication intake, (III) no definite inflammation or infection signs or symptoms at the time of surgery, and (IV) no radiological abnormalities except pectus excavatum, such as tumors or pneumonia. Pectus BR was usually conducted 2 to 3 years after NP, based on patient condition. The laboratory tests on total LDH activity and its isoenzyme patterns were routinely conducted the day before surgery when blood samples were obtained for routine preoperative laboratory checkup. The references of total LDH and its isoenzyme pattern are determined through standard methods, including healthy people for regular examinations and outside data accumulation (10). No patient data was discarded on sampling or test errors. Values and patterns on LDH isoenzyme were separated and obtained by electrophoretic methods, and all laboratory results were obtained by standard methods using an auto analyzer (Hitachi 7600-210; Hitachi, Tokyo, Japan) and available assay kits (Sekisui Medical Co., Ltd., Tokyo, Japan). In order to investigate total LDH and its isoenzyme patterns in patients with pectus excavatum, we analyzed (I) total LDH and its isoenzyme patterns before correction (compared with the reference values), (II) relationships of total LDH and its isoenzymes with age at time of NP, sex, severity of pectus excavatum, and pectus morphology types, and (III) post-corrective changes.
Statistical considerations and ethical statement
All data are presented as the mean ± SD. To judge the laboratory findings as normal or abnormal, data were compared with the lower or upper limit of the reference range using one sample t-tests. Because data distribution was non-normalized, nonparametric statistical methods (the Wilcoxon signed-rank test and the Spearman correlation test) were used when comparisons were performed. The Chi-Square statistic was used to check relationships between categorical variables. A multivariate linear regression model (backward, stepwise approach) was used to investigate independent parameters of total LDH and its isoenzyme patterns on pectus excavatum. The results were analyzed using the Statistical Package of Social Sciences version 22.0 (SPSS, IBM Corp, NY, USA), with a value of significance of 0.05. An approval from Uijeongbu Saint Mary’s Hospital Ethics Committee was obtained for this study (approval number: UC19RESI0082).
Results
A total of 85 patients were included in the study. The mean age of study subjects was 13.6±6.5 years at the age of the NP and the mean interval between NP and BR was 2.2±0.42 years. Seventy-one males and 14 females were included. The pectus types included 54 symmetric and 31 asymmetric cases. The mean Haller index before NP and BR was 3.8±1.45 and 2.7±0.4, respectively. The mean total LDH before NP (pre-correction) and BR (post-correction) was 404.2±80.8 and 369.2±79.3IU/L, respectively. The overall clinical characteristics of the study subjects are presented in Table 1.
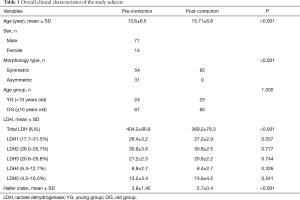
Full table
Pre-corrective total LDH activity and its isoenzyme patterns
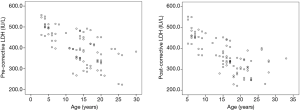
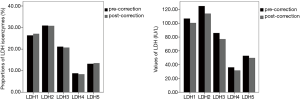
Because the reference range for total LDH is different by age, we divided subjects into two groups (young group, YG <10 years old, and old group, OG ≥10 years old) (10). Total LDH in both groups was significantly higher than the normal values (YG, P=0.006, and OG, P<0.001) (Figure 1). LDH isoenzyme patterns are shown in Figure 2. There was a significant difference in LDH isoenzyme patterns from the reference. In other words, the proportion of LDH5 was significantly higher than LDH4 (P<0.001) while the proportion of LDH4 is significantly higher than LDH5 in normal populations. However, proportions of each isoenzyme were all within the reference range.
Relationships of total LDH and its isoenzymes with various factors of pectus excavatum
We assumed that age at time of NP, sex, severity of pectus excavatum, and pectus morphology types were associated with total LDH and its isoenzyme patterns. Severity was defined with the Haller index (11). Multivariate analysis using a linear regression model analysis was performed to investigate the independent influencing factors for total LDH level in patients with pectus excavatum. The analysis showed that total serum LDH was significantly associated with age at time of NP and the Haller index (P<0.001 and P=0.030). We divided the subjects into two groups according to each influencing factor (age at time of NP and the Haller index).
Ages at time of surgery
We divided the subjects into two groups by age at the time of surgery (YG <10 years old and OG ≥10 years old). Total LDH in the YG was significantly higher than in the OG (<0.001) and there was no significant difference in LDH isoenzyme patterns between them (Table 2).
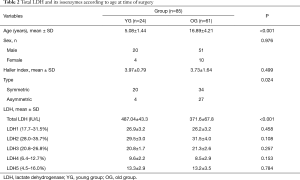
Full table
Severity
There was no significant correlation between severity and total LDH. However, values of only LDH5 among all isoenzymes had a significant positive correlation with severity (P=0.006). In addition, we divided the subjects into two groups by the median values of Haller index (3.5). We defined the high group (HG) as having Haller index >3.5. There was no significant difference in total LDH according to severity and the proportion of only LDH5 among all the isoenzymes was significantly higher in the HG (P=0.003) (Table 3).
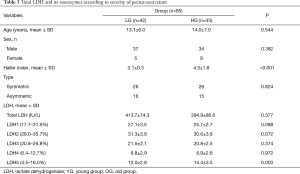
Full table
Post-corrective changes of total LDH activity and its isoenzyme patterns
There was a significant decrease of total LDH after correction (P<0.001), and post-corrective total LDH was not significantly different from than normal value. LDH isoenzyme patterns are shown in Figure 2. There was also a significant difference in LDH isoenzyme patterns with the normal patterns and there were no post-corrective changes of LDH isoenzyme patterns. The proportion of LDH5 was also significantly higher than LDH4 after correction (P<0.001) (Figure 2). In addition, proportions of each isoenzyme were within the reference range, however, there were significant decreases in values of LDH1–LDH4, except LDH5 (P=0.020, P<0.001, P<0.001, and P=0.029) (Table 4).

Full table
Discussion
Generally, patients with pectus excavatum are healthy, nearly asymptomatic, and are not known to show any specific laboratory findings so that laboratory study is considered unnecessary (1,6). However, we showed that pectus excavatum has various effects on the whole body, such as body growth and spinal scoliosis (2,12,13). In addition, some severe cases of pectus excavatum present with jaundice and right heart failure (1,5). Therefore, we assumed that the disease entity or compressions of internal organs could affect serum laboratory findings and we showed elevation of serum total LDH and decrease of total LDH after correction of the deformity (6). In our previous study, we hypothesized that total LDH elevation before correction could be due to a combination of disease entity (musculoskeletal disorder) and compression of internal organs and that total LDH decrease after correction can be due to alleviation of internal organ compressions (6). However, studies on LDH isoenzyme patterns were needed to verify this hypothesis. In the present study, we performed an LDH isoenzyme study in patients with pectus excavatum in order to investigate possible causes. To the best of our knowledge, this study is the first to undertake an LDH and its isoenzymes study in patients with pectus excavatum.
This study had four findings. The first, like our previous study, showed that total LDH was significantly higher than the normal limit pre-correctively and decreased to the normal value post-correctively. Some studies have reported that total LDH is elevated in certain thoracic conditions, such as myocardial infarction, heart failure, and pneumonia (7,8,10). Total LDH can also be elevated in pectus excavatum by our above-mentioned hypothesis. In addition, multivariate analysis showed that value of total LDH was associated with age at the time of surgery and severity, which also showed that total LDH elevation was associated with pectus excavatum. The second finding was that LDH isoenzyme pattern was different from the normal one. The proportion of LDH5 was significantly higher than LDH4 in patients with pectus excavatum while LDH isoenzymes in the normal population was ordered as follows: LDH2 > LDH1 > LDH3 > LDH4 > LDH5 (8). As flipping of LDH isoenzyme (LDH1 > LDH2) is well-known to be helpful in diagnosis of acute myocardial infarction, a proportion of LDH5 higher than LDH4 was found in pectus excavatum (9). The proportion of LDH5 higher than LDH4 suggests that the cause of LDH elevation is associated with a disease of muscular origin, which also suggests that pectus excavatum is a muscular disease entity (3). However, other enzymes associated with muscular disease were interestingly within normal ranges, which were possibly assumed to be associated with chronicization of pectus excavatum (9). In addition, this finding can be associated with concomitant heritable disorders of connective tissue in pectus excavatum (3,14). The third finding was that the proportion of only LDH5 was significantly positively correlated with severity and the proportion of only LDH5 was significantly higher in the severe group. This finding also shows that patients with pectus excavatum have a muscular disorder. However, there was no association between total LDH and other isoenzymes (LDH1–LDH4) and severity of pectus excavatum. One of the causes was considered to be a lack of methods to fully describe the degree of internal organ compression (11,15). If exact degree of compression on each organ could be evaluated, the association between LDH and its isoenzymes and severity of pectus excavatum could be presented. The fourth finding was that there were no significant changes in patterns of LDH isoenzyme (proportion of each isoenzyme) after correction and there were significant decreases in values of LDH1–LDH4, except LDH5. These findings suggest alleviation of internal organ compression, which provided the LDH1–LDH4 decrease, and that the disease entity itself did not change after correction.
This study has a few limitations. Firstly, it was retrospective with a small sample size conducted at a single center. Larger scale and multi-institutional studies are required to verify these findings. Secondly, although severity of pectus excavatum is considered to be associated with LDH activity or its isoenzyme pattern, we could not show the correlation of severity with LDH activity and its isoenzyme pattern. We assume that one of the causes is that because no methods or tools exist to fully describe chest wall or internal organ compression (11,15). These methods are needed to fully describe the pectus excavatum.
Conclusions
In summary, we showed that pectus excavatum was associated with total LDH activity and its isoenzyme patterns. This study showed that pectus excavatum is a muscular disease entity and that laboratory findings are also associated with compression of internal organs, which was explained by post-corrective changes in LDH activity and isoenzyme pattern. This study will provide a deeper and wider comprehension of pectus excavatum (3).
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. An approval from Uijeongbu Saint Mary’s Hospital Ethics Committee was obtained for this study (approval number: UC19RESI0082) and written informed consent was obtained from all patients.
References
- Al-Qadi MO. Disorders of the Chest Wall: Clinical Manifestations. Clin Chest Med 2018;39:361-75. [Crossref] [PubMed]
- Park HJ, Kim JJ, Park JK, et al. A cross-sectional study for the development of growth of patients with pectus excavatum. Eur J Cardiothorac Surg 2016;50:1102-9. [Crossref] [PubMed]
- Brochhausen C, Turial S, Müller FK, et al. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg 2012;14:801-6. [Crossref] [PubMed]
- Koumbourlis AC. Pectus excavatum: pathophysiology and clinical characteristics. Paediatr Respir Rev 2009;10:3-6. [Crossref] [PubMed]
- Nuss D, Obermeyer RJ, Kelly RE. Nuss bar procedure: past, present and future. Ann Cardiothorac Surg 2016;5:422-33. [Crossref] [PubMed]
- Kim JJ, Kim CK, Park HJ, et al. Elevation of serum lactate dehydrogenase in patients with pectus excavatum. J Cardiothorac Surg 2014;9:75. [Crossref] [PubMed]
- Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett 2015;358:1-7. [Crossref] [PubMed]
- Drent M, Cobben NA, Henderson RF, et al. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J 1996;9:1736-42. [Crossref] [PubMed]
- Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med 2010;48:757-67. [Crossref] [PubMed]
- Erez A, Shental O, Tchebiner JZ, et al. Diagnostic and prognostic value of very high serum lactate dehydrogenase in admitted medical patients. Isr Med Assoc J 2014;16:439-43. [PubMed]
- Sesia SB, Heitzelmann M, Schaedelin S, et al. Standardized Haller and Asymmetry Index Combined for a More Accurate Assessment of Pectus Excavatum. Ann Thorac Surg 2019;107:271-6. [Crossref] [PubMed]
- Kim JJ, Park HJ, Park JK, et al. A study about the costoclavicular space in patients with pectus excavatum. J Cardiothorac Surg 2014;9:189. [Crossref] [PubMed]
- Park HJ, Kim JJ, Park JK, et al. Effects of Nuss procedure on thoracic scoliosis in patients with pectus excavatum. J Thorac Dis 2017;9:3810-6. [Crossref] [PubMed]
- Tocchioni F, Ghionzoli M, Messineo A, et al. Pectus excavatum and heritable disorders of the connective tissue. Pediatr Rep 2013;5:e15. [Crossref] [PubMed]
- Kim M, Lee KY, Park HJ, et al. Development of new cardiac deformity indexes for pectus excavatum on computed tomography: feasibility for pre- and post-operative evaluation. Yonsei Med J 2009;50:385-90. [Crossref] [PubMed]


