
The probability of nodal metastasis in novel T-factor: the applicability of sublobar resection
Introduction
A number of studies on the validity of sublobar resection for peripheral small-sized non-small cell lung cancer (NSCLC) have focused on the total tumor diameter and the ratio of the total tumor diameter to the consolidation area (consolidation/tumor ratio: C/T ratio) on computed tomography (CT), since the C/T ratio has been considered a key feature of invasiveness (1). The Japan clinical oncology group (JCOG) has conducted 3 prospective phase 3 trials, the JCOG0804/WJOG4507L (wide wedge resection for a C/T ratio of <25% and a tumor diameter of <2 cm), JCOG1211 (segmentectomy for a C/T ratio of 25–50% and a tumor diameter of <2 cm, or a C/T ratio of <50% and a tumor diameter of 2–3 cm), and JCOG0802/WJOG4607L (segmentectomy for a C/T ratio of 50–100% and a tumor diameter of <2 cm) trials (2). Recently, the final results of the JCOG0804/WJOG4507L trial were reported at the Annual meeting of the American Society of Clinical Oncology 2017 (3). The results showed that tumors that met the abovementioned conditions can be curatively treated by wedge resection. In addition, several database studies indicated that appropriately selected NSCLC tumors of ≤2 cm in diameter would be good candidates for sublobar resection that included both wedge resection and segmentectomy (4-6).
A previous study indicated that tumors with ground glass opacity (GGO) on CT showed a favorable prognosis in comparison to tumors without GGO (pure-solid) (7). The radiologic GGO area of lung adenocarcinoma corresponds to the non-invasive area on pathological specimens (1,8), and a tumor accompanied by GGO may suggest the lining growth pattern in the alveolar space. In this sense, the biology of NSCLC tumors with and without GGO may be quite different. In the new version 8 staging system (9,10), the ground glass portion was finally omitted from the tumor diameter for the consideration of the T-factor. Thus, it is important to determine how the new T-factor should be applied when identifying candidates for sublobar resection.
Nodal metastasis is one of the most important prognostic factors for lung cancer (11,12), and surgical pathology sometimes reveals nodal metastasis in patients with clinical N0 NSCLC (13). The prognosis of patients with N2 remains poor with a 5-year survival rate of 37% (14). A previous report also emphasized the importance of a nodal survey, even when performing sublobar resection (6). In this study, the relationship between the new T-factor and nodal metastasis in peripheral small-sized lung cancer was examined with the aim of improving the selection of candidates for sublobar resection (wedge resection or segmentectomy).
Methods
Ethical approval
The ethics committee of Chiba University Graduate School of Medicine approved this research (No. 2652 and 3033).
Patients
From January 2013 to October 2017, a consecutive series of 545 patients with cT1 or cTis lung cancer [TNM classification, 8th edition (9)] who were undergoing pulmonary resection was evaluated, and their clinical and pathological data were collected from a prospective surgical database that was maintained by our department. Patients with the following conditions were excluded from the analysis: non-peripheral type, induction treatment, clinical N1 or more, clinical M1, no nodal dissection, no available radiological imaging of thin-sliced computed tomography or fluorodeoxy glucose-positron emission tomography (FDG-PET).
The radiologic maximum size and location of tumors were defined by thin-sliced CT, which involved multi-dimensional slicing and reconstruction into axial, coronal, and sagittal views. The tumor location was also classified into three loci based on three-dimensional imaging: peripheral type was defined as being located in the outer third layer of the whole lung. The C/T ratio was calculated based on the measured maximum tumor size (including GGO) and the consolidation size. Accordingly, the tumor CT findings were assigned to one of the following three groups: pure GGO (C/T ratio =0%), mixed GGO (C/T ratio >0 to <100%, and solid-type (C/T ratio =100%). Clinical information was collected from database. These prospectively collected clinical and pathological data were retrospectively analyzed to investigate the relationships with the clinical T factor and the pathological N factor.
Statistical analysis
The frequency analysis was performed using the chi-squared test. Data were analyzed using the JMP software program (version 10, SAS Institute Inc., Cary, NC, USA). All P values were based on a two-tailed hypothesis test; P values of <0.05 were considered to indicate statistical significance.
Results
Patient characteristics
The characteristics of eligible patients and their lesions are summarized in Table 1. A total of 325 patients (male, n=196; female, n=129) were eligible for inclusion in this study. The average patient age was 68.2±8.8 years. The CT findings of the tumors were as follows: pure GGO (n=12; 3.7%), mixed GGO (n=123; 37.8%), and solid type (n=190; 58.5%). The median maximum standardized uptake value (SUVmax) of the primary tumor on preoperative FDG-PET was 2.98 (range, 0–24.4). Lobectomy with nodal dissection was performed in 246 patients (75.7%). The preoperative radiological staging of this cohort resulted in the following classifications: cTis (n=10), cT1mi (n=11), cT1a (n=51), cT1b (n=146), and cT1c (n=107).
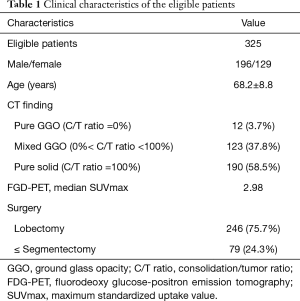
Full table
Pathological findings
Pathologic nodal metastasis was observed in 38 patients (11.7%), including 21 patients with pN1 and 17 patients with pN2, within this cN0 cohort. The pathological classifications of the cohort were as follows: adenocarcinoma (n=253), squamous cell carcinoma (n=52), large or small cell carcinomas (n=16; including 8 combined small cell carcinomas), and other rare cell types (n=4). With regard to the specific cell types, 11.1% (n=28) of the patients with adenocarcinoma, 9.6% (n=5) of the patients with squamous cell carcinoma, and 25.0% (n=4) of the patients with large or small cell carcinoma showed nodal metastasis. pN2 metastasis was frequently observed in patients with adenocarcinoma (n=14). The detailed pathological findings are summarized in Table 2.
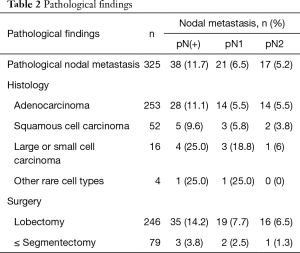
Full table
The clinical T factor and the pathological N factor
In UICC version 8, no nodal metastasis was found in the surgical pathology of 21 patients of cTis or cT1mi. Three of the patients with clinical T1a (5.9%; including one patient with pN2) showed nodal metastasis. Two pN1 metastases were observed at the #11s node from the right upper lobe (16.8 mm tumor in S3, C/T ratio =53.6%) and the #12l node from the right lower lobe (5.0 mm tumor in S8, C/T ratio =100%). Seventeen patients with clinical T1b (11.6%) and 18 patients with T1c (16.8%) showed nodal metastasis. Notably, pN2 metastasis was confirmed in 8 patients of each cohort (4.5% of cT1b, and 7.5% of cT1c). Clinical T1b and T1c patients showed significantly high rates of nodal metastasis (13.4% vs. 4.2%, P=0.024) in comparison to patients with earlier cT (Tis, T1mi, and T1a): they also tended to show a higher rate of pN2 metastasis (6.3% vs. 1.4%, P=0.097).
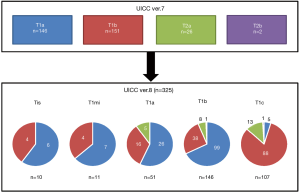
On the other hand, once the clinical T factor from UICC version 8 was converted to that of version 7 (Figure 1). The classifications of this cohort were as follows: cT1a (n=146), cT1b (n=151), cT2a (n=26) and cT2b (n=2). Thus, the classifications of the population according to UICC versions 7 and 8 were quite different. The rates of nodal metastasis according to the classifications were as follows: cT1a, 10.3%; cT1b, 13.9%; cT2a, 7.7%; and cT2b, 0%; the rates did not differ to a statistically significant extent (P=0.64). The numbers of patients with pathological N2 disease, according to the version 7 classifications, were as follows: cT1a (n=6), cT1b (n=9), cT2a (n=2) and cT2b (n=0); the distribution did not differ to a statistically significant extent (P=0.81).
The prediction of pathological nodal metastasis in cT1b/c patients
Focusing on the cT1b and cT1c patients, pathological nodal metastasis was found in 35 of 235 patients. No patients with pure GGO tumors (no pathological nodal metastasis) were included in this sub-cohort. Solid-type tumors were seen in 177 patients, 28 of whom (15.8%) showed pathological nodal metastasis; in contrast, nodal metastasis was found in 7 of 76 patients with mixed GGO-type tumors (9.2%) (P=0.16). The 35 patients with nodal metastasis showed significantly higher SUVmax in comparison to the other 200 patients (median 6.0 vs. 3.5, P=0.0118). A receiver operating characteristic (ROC) curve analysis revealed that the cutoff SUVmax for the prediction of nodal metastasis in cT1b/c patients was 4.54; the area under the curve (AUC), sensitivity and specificity were 0.632, 0.686 and 0.596, respectively. Even though pN2 (n=16) cases, showed relatively high SUVmax, the difference was not statistically significant (median 5.9 vs. 3.7, P=0.15).
Among patients with solid-type tumors, the SUVmax on preoperative FDG-PET was similar in pN1/2 (n=28) and pN0 (n=149) patients (median 6.3 vs. 5.2, P=0.3). In contrast, the SUVmax of mixed GGO tumors with pN0 (n=69) was significantly lower in comparison to mixed GGO with pN1/2 (n=7) (1.6 vs. 4.8, P=0.0045). A ROC curve analysis revealed that the cutoff SUVmax for pN0 in the T1b/c patients was 1.9 (AUC=0.8271, sensitivity=1.0, specificity=0.594).
Thus, utilizing the combination of mixed GGO type and SUVmax <1.9 can exclude patients with pathological nodal metastasis (P=0.0051) from cT1b/c patients (Table 3).
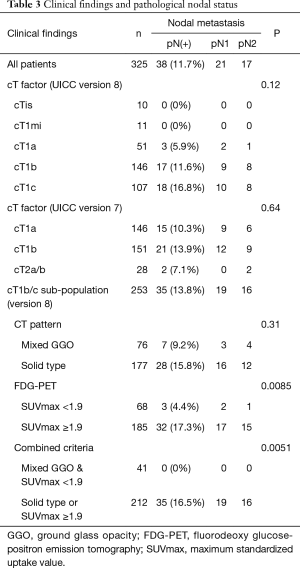
Full table
Discussion
In this paper, we considered pathological nodal status to be a noteworthy factor when deciding the indications for intentionally limited surgery. Our data indicated some clinical clues for the assessment of patients for intentionally limited surgery. From the present study, cTis and cT1mi tumors are considered to show non-invasive radiological features with no nodal metastasis; thus, patients with these tumors are considered to be candidates for sublobar resection. On the other hand, T1a/b/c are assumed to include a relatively high proportion of invasive tumors. The proportions of patients with these types who showed nodal metastasis were as follows: cT1a (5.9%), cT1b (11.6%) and cT1c (16.8%). These results clearly indicate that the new TNM system of version 8 is still insufficient for predicting nodal metastasis; however, the consideration of the invasiveness of the tumor is reflected in the T factor by measuring the size of the solid component of tumor. We focused on the CT findings and SUVmax on FDG-PET. This clinical information can be obtained preoperatively and can be used in the indication for limited surgery. It was reported that the existence of a GGO component in the tumor might reflect less-invasiveness and a good overall prognosis (15); however, the analysis of the cT1b/c patients revealed that a solid CT appearance was not sufficient for excluding patients with potential nodal metastasis [80.0% (28/35), P=0.31]. FDG-PET is a strong tool for the qualitative and quantitative evaluation of the aggressive behavior of lung cancer, even though it shows a low positive predictive value for the diagnosis (16,17). The possible clinical application of FDG-PET in relation to decision-making with regard to the surgical strategy was previously reported in stage IA lung adenocarcinoma (18). Our data revealed that patients with lung cancer with nodal metastasis showed significantly high SUVmax (P=0.0118), but that it was not appropriate to apply the cutoff for the prediction of nodal metastasis, due to the low positive predictive value. Thus, we selected 1.9 as the negative predictive cutoff value (positive cutoff value for pN0), which excluded most cases involving nodal metastasis [91.4% (32/35) in cT1b/c cohort]. Accordingly, the combined criterion of the CT feature (mixed GGO type) and a SUVmax on FDG-PET (SUVmax <1.9) could eliminate all patients with nodal metastasis [100% (35/35) in the cT1b/c cohort], and may become an acceptable indication for the limited surgery. In this retrospective analysis, pathological nodal metastasis was seen in the cT1a/b/c cohort and metastasis was sometimes observed on N2 lesions.
A previous retrospective study on the indications for limited resection focused on non-small cell carcinoma, especially adenocarcinoma (19). In the clinical setting, however, it is often difficult to obtain a definite preoperative diagnosis of peripheral small lesions; then we cannot be sure that the objects of the present study are limited to adenocarcinoma. Our study revealed tumors with GGO observed in 52.6% of adenocarcinoma (133/253) and 3.9% of squamous cell carcinoma (2/52). Other all 20 lung cancers (small cell and large cell lung cancer) were of the solid type, which were not diagnosed preoperatively. Thus, special attention should be paid when performing limited resection for solid-type tumors and nodal dissection should be performed.
In practice, almost one fourth of eligible patients underwent sublobar resection with nodal dissection in this study (Table 1). With the application of the CT and FDG-PET criterion, 22.4% (55/246) of lobectomy patients were potential candidates for limited resection, and 59.4% (47/79) of the segmentectomy patients were regarded as good candidates for limited nodal dissection.
Once the relationship between the tumor size, C/T ratio, and nodal metastasis is visualized (Figure 2), it is clear that the change in the T-factor definitions from UICC version 7 to 8 affected the correlation between the clinical T factor and the pathological N factor. Theoretically, the size of consolidation (equivalent to the invasive portion) of the tumor could be correlated with disease progression (1), including the possibility of nodal metastasis. In version 7, even the earliest T factor (T1a) showed a relatively high incidence of nodal metastasis; thus, this T factor is not useful for the prediction of nodal metastasis. On the other hand, when the patients were classified according to version 8, the patients with cTis and cT1mi showed no nodal metastasis, and nodal metastasis gradually increased according as the cT factor increased. Notably, the distribution of nodal metastasis cases was scattered for most cases with a C/T ratio of 100% (solid-type tumors). As Figure 2 illustrated, version 8 superior to version 7 for classifying the aggressiveness of tumors.
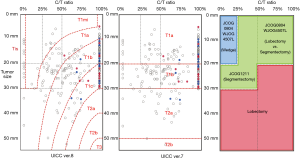
Interestingly, some patients in ongoing clinical trials of limited surgery for peripheral small-sized lung cancer (2) could also be allocated (right part of Figure 2). The JCOG0804/WJOG4507L trial is validating the efficacy of wedge resection (blue box) in T1a (version 7) patients with a C/T ratio <25%. This target population included patients with cTis and cT1mi (version 8), who—based on our results—would seem to be good candidates for sublobar resection; as no nodal metastasis was observed among such patients, complete resection could be achieved by wedge resection, as a sufficient surgical margin could be maintained. Recently, this was demonstrated by the final results of the JCOG0804/WJOG4507L trial (3). The JCOG0802/WJOG4607L trial focused on a C/T ratio of >50% in T1a (version 7) patients (right side of the green box) after the revision of the enrollment criteria in 2013. This phase III study compared lobectomy and segmentectomy. The cohort mainly consisted of patients with cT1mi/a/b. The point of the sub-cohorts was to include tumors with a C/T ratio of 100% as solid-type tumors, which sometimes show nodal metastasis. Our study suggests that not only the surgical margin but also an examination to detect lymph node metastasis (including an investigation of the mediastinal nodes) should be considered for patients with such tumors. The enrollment in this trial was completed in 2014. The JCOG1211 trial is a single-arm non-randomized confirmatory trial to validate the efficacy of segmentectomy in T1a (version 7) tumors with a C/T ratio of 25–50% and T1b (version 7) tumors with a C/T ratio <50% (left side of green box). As illustrated in Figure 2, this population is broadly spread from Tis, 1mi, 1a, and a small part of 1b. Importantly, our study indicated no nodal metastasis in these patients, and the previous investigation also indicated that tumors with a C/T ratio of <50% were less invasive (7). Moreover, patients with a C/T ratio of <50% were reported to show satisfactory prognoses (20). Enrollment in this trial was completed in 2015. Through our study, lung cancer with a total tumor diameter of >3 cm and C/T ratio of <50%, which would be classified as cT1a/b/c (left side of pink box), showed no nodal metastasis; thus, if a sufficient surgical margin can be ensured, such cases might become candidates for limited surgery.
Our study is associated with some study limitations. First, only 59.6% (325/545) of the cT1 or cTis patients were evaluated in this study. Our study’s target population was patients with early stage lung cancer. In this population, limited surgery is sometimes selected for reasons such as a poor respiratory function or due to the presence of severe comorbidities. Nodal dissection was omitted in some of the patients who were excluded. This selection bias could lead to the underestimation of the incidence of nodal metastasis. Second, 24.3% of the patients in the enrolled population underwent segmentectomy with nodal dissection. Hilar and mediastinal nodal dissection was performed in these cases, although limited surgery cannot prevent the occurrence of intrapulmonary nodal metastasis to “non-tumor bearing segment”. We previously reported that inter-segmental metastasis was a very rare event except in cases without hilar nodal metastasis (21); this hypothesis was investigated in cadaver study by other investigators (22). For the reasons mentioned above, we considered these limitations to be acceptable. Thirdly, this study is single institutional retrospective study. The patient selection, the determination of the operative method, and the surgical indications might have included biases. To overcome this limitation, a multi-institutional prospective study should be performed.
Conclusions
Sublobar resection without nodal dissection would be a therapeutic option for cTis/1mi NSCLC, classified according to the UICC (8th edition). Lobectomy and nodal dissection are still indicated for cT1a/b/c, however, sublobar resection with nodal dissection can be indicated for cT1a/b/c patients if specific conditions are applied, namely a tumor with GGO on CT and a low SUVmax (<1.9).
Acknowledgments
All authors have read and approved the final version of this manuscript. We sincerely thank all of the thoracic surgeons in the Department of General Thoracic Surgery, Chiba University Hospital, for their cooperation in data collection.
Footnote
Conflicts of Interest: This data was presented at 25th European Conference on General Thoracic Surgery, Innsbruck, Austria, May 28-31, 2017.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethics committee of Chiba University Graduate School of Medicine approved this research (No. 2652 and 3033).
References
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Aokage K, Yoshida J, Hishida T, et al. Limited resection for early-stage non-small cell lung cancer as function-preserving radical surgery: a review. Jpn J Clin Oncol 2017;47:7-11. [Crossref] [PubMed]
- Suzuki K, Watanabe S, Wakabayashi M, et al. A nonrandomized confirmatory phase III study of sublobar surgical resection for peripheral ground glass opacity dominant lung cancer defined with thoracic thin-section computed tomography (JCOG0804/WJOG4507L). J Clin Oncol 2017;35:abstr 8561.
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: A multicenter study. J Thorac Cardiovasc Surg. 2006;132:769-75. [Crossref] [PubMed]
- Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J. 2009;33:426-35. [Crossref] [PubMed]
- Speicher PJ, Gu L, Gulack BC, et al. Sublobar Resection for Clinical Stage IA Non-small-cell Lung Cancer in the United States. Clin Lung Cancer 2016;17:47-55. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic Classification of Small Adenocarcinoma of the Lung: Radiologic-Pathologic Correlation and Its Prognostic Impact. Ann Thorac Surg 2006;81:413-9. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Detterbeck FC, Chansky K, Groome P, et al. The IASLC Lung Cancer Staging Project: Methodology and Validation Used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1433-46. [Crossref] [PubMed]
- Yoshino I, Yoshida S, Miyaoka E, et al. Surgical outcome of stage IIIA- cN2/pN2 non-small-cell lung cancer patients in Japanese lung cancer registry study in 2004. J Thorac Oncol 2012;7:850-5. [Crossref] [PubMed]
- Ichinose Y, Kato H, Koike T, et al. Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: questionnaire survey of the Japan Clinical Oncology Group to plan for clinical trials. Lung Cancer 2001;34:29-36. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. The prognosis of surgically resected N2 non-small cell lung cancer: the importance of clinical N status. J Thorac Cardiovasc Surg 1999;118:145-53. [Crossref] [PubMed]
- Yang CF, Kumar A, Gulack BC, et al. Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis. J Thorac Cardiovasc Surg 2016;151:1380-8. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Prognostic impact of a ground glass opacity component in the clinical T classification of non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:2102-2110.e1. [PubMed]
- Minamimoto R, Senda M, Jinnouchi S, et al. Detection of lung cancer by FDG-PET cancer screening program: a nationwide Japanese survey. Anticancer Res 2014;34:183-9. [PubMed]
- Perigaud C, Bridji B, Roussel JC, et al. Prospective preoperative mediastinal lymph node staging by integrated positron emission tomography-computerised tomography in patients with non-small-cell lung cancer. Eur J Cardiothorac Surg 2009;36:731-6. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Prediction of pathologic node-negative clinical stage IA lung adenocarcinoma for optimal candidates undergoing sublobar resection. J Thorac Cardiovasc Surg 2012;144:1365-71. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Indications for sublobar resection of clinical stage IA radiologic pure-solid lung adenocarcinoma. J Thorac Cardiovasc Surg 2017;154:1100-8. [Crossref] [PubMed]
- Aokage K, Miyoshi T, Ishii G, et al. Influence of Ground Glass Opacity and the Corresponding Pathological Findings on Survival in Patients with Clinical Stage I Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:533-42. [Crossref] [PubMed]
- Sakairi Y, Yoshino I, Yoshida S, et al. Pattern of Metastasis Outside Tumor-bearing Segments in Primary Lung Cancer: Rationale for Segmentectomy. Ann Thorac Surg 2014;97:1694-700. [Crossref] [PubMed]
- Topol M, Masłoń A. Some variations in lymphatic drainage of selected bronchopulmonary segments in human lungs. Ann Anat 2009;191:568-74. [Crossref] [PubMed]


