Nonspecific interstitial pneumonia and usual interstitial pneumonia: comparison of the clinicopathologic features and prognosis
Introduction
Idiopathic nonspecific interstitial pneumonia (NSIP) has been recently named as a histological type of idiopathic interstitial pneumonia (IIP). Interstitial pneumonia that cannot be categorized is referred to as NSIP, which includes three subtypes. However, there are questions regarding the possible similarities between NSIP and usual interstitial pneumonia (UIP). Comparative histopathologic studies about these two conditions have rarely been conducted and reported worldwide (1,2), and the distinctions between them remains unclear. Therefore, in the present study, the clinical, pathologic, and follow-up findings in NSIP and UIP patients who underwent open surgical or video-assisted thoracoscopic lung biopsy and treatment over a 10-year duration at our institution were reviewed. Furthermore, the differential diagnosis of NSIP and UIP, and the potential mechanisms of NSIP were discussed.
Materials and methods
Patients and setting
The medical records of 121 patients with diffuse lung disease (suspected as IIP) examined and underwent video-assisted thoracoscopic surgery (VATS) or open surgical lung biopsy at Shanghai Pulmonary Hospital (affiliated hospital of Tongji University) between March 1999 and February 2005 were reviewed. In the 121 cases, 21 were diagnosed with UIP, and 29 were diagnosed with NSIP. A total of 18 UIP patients and 21 NSIP patients who had a complete clinical history and were followed up for at least 1 year postoperatively were analyzed.
Patients were diagnosed with UIP or NSIP according to the IIP diagnostic criteria described by the American Thoracic Society (ATS) and European Respiratory Society (ERS) in 2000 and 2002 (1,3). All final diagnoses were made by consensus of pulmonologists, pathologists, and radiologists (clinical-radiologic-pathologic diagnosis). The study was approved by the institutional review board. The requirement for informed consent was waived.
Treatment and follow-up
Glucocorticoids and symptomatic treatment were administered in 39 patients; 11 UIP patients and 5 NSIP patients were also administered azathioprine (4). Chest radiography and high-resolution computed tomography (HRCT) were performed at 1, 3, 6, 9, and 12 months after initiating treatment. Therapeutic success was determined based on clinical symptoms and signs, such as improvement, stabilization, or deterioration. Improvement and stabilization were considered signs of treatment effectiveness. The criteria used to define improvement were as follows: no overt symptoms, improved respiratory function, resolution of lesions on imaging, stable condition, and no evidence of disease recurrence. Stabilization was considered on the basis of the following findings: resolution of symptoms, mild improvement in respiratory function, partial, or no absorption of lesions on imaging, or disease recurrence. The treatment was considered invalid when symptoms did not resolve and lesions showed no absorption on imaging.
Statistical analysis
Data were analyzed using the SPSS 10.0 software package. Data between the patient groups were compared using χ2 test (chi-square test). Statistical significance was designated at P<0.05.
Results
Patient demographics and clinical findings
The mean patient age in the UIP group was 60 years and ranged from 50-75 years; the group comprised 12 men and 6 women. Thirteen patients had a history of smoking, and 6 patients had previous contact with inorganic or organic dust. Previous exposure ranged from 5-62 months and averaged 21.6 months. In the NSIP group, the mean patient age was 48 years and ranged from 28-70 years; the group included 7 men and 14 women. Eight patients had a history of smoking, and four patients had previous contact with inorganic or organic dust. Previous exposure ranged from 1-24 months and averaged 7.8 months.
The clinical findings in the UIP and NSIP patients are summarized in Table 1. Bacterial culture of sputum yielded negative results in all patients. Anti-nuclear antibody and molecular reactions yielded negative results in all patients except one patient in the NSIP group, who had increased O antibody levels. In the UIP patients, the chest radiographs showed asymmetric bilateral reticular shadows in the basal and peripheral lungs, and decreased lung volume. The CT and HRCT showed flake- and net-like shadows, primarily in the basal lungs. In a few patients, ground-glass shadows and severe fibrosis were present and were accompanied by traction bronchiectasis, bronchiolectasis, or subpleural honeycomb-like lesions.
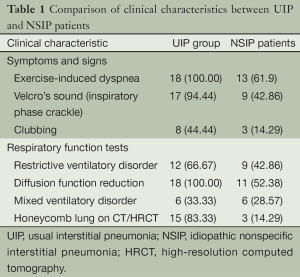
Full table
In the NSIP patients, the chest radiographs showed interstitial diffuse infiltration with net-like and fibrous linear pulmonary opacities. The CT and HRCT images showed varying severities of ground-glass shadows, and net-like and fibrous linear pulmonary opacities, with no honeycomb-like changes. Based on the history of occupational exposure, clinical manifestation, laboratory examination, and histopathology (including polarizer observation), collagen vascular disease, occupational pneumoconiosis, drug-induced interstitial lung disease, and infection were excluded.
Histopathologic examination
An incisional biopsy was performed in 4 patients of the UIP group and 8 patients of the NSIP group; the remainder underwent a VATS lung biopsy. In each procedure, at least three samples were taken from mildly, moderately, and severely diseased tissues, which were ≥1.0 cm × 0.7 cm × 0.5 cm.
Histopathology in the UIP group
At low magnification, the lesions varied in severity and were distributed erratically. Chronic and acute lesions of interstitial inflammation, fibrosis, and honeycombing were interspersed among the normal lung tissue, and were primarily within the subpleural lung parenchyma. The interstitial inflammation was typically patchy with alveolar septum infiltrates comprising leukomonocytes and plasmacytes accompanied by type II alveolar cell proliferation. Diffuse hyperplastic fibrous tissue with collagen deposition formed the alveolar structure. In the areas of inflammation, fibrosis, and honeycomb changes, foci in a light-blue myxoid stroma background were observed. These foci comprised proliferative fibroblast and myoblast cells were identified as myofibroblast cell foci. The honeycombed lung formed by a cystic fiber chamber was often covered by bronchial epithelial cells and contained mucus. In the fibrotic and honeycomb areas, smooth muscle proliferation was observed, which was patchy and at times with myogelosis. In two cases, mixed diffuse alveolar damage, cell proliferation, and the loss of the alveolar epithelial cells were also present. The lung interstitium also showed fibroblast proliferation and a macrophagocyte mass along with serous effusion from the alveolar space. The incidences of each particular lesion in both patient groups are summarized in Table 2.
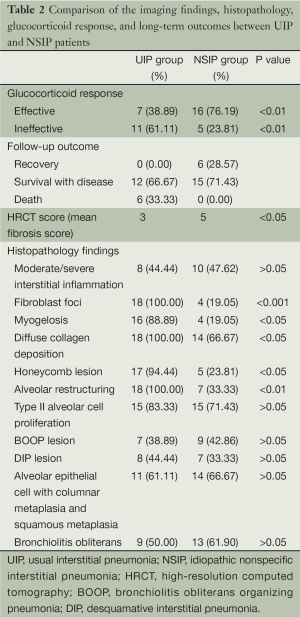
Full table
Histopathology in the NSIP group
All cases showed varying severities of chronic inflammation and interstitial fibrosis. The pathological changes were similar across the tissue samples. On histologic examination, the subtypes were distributed as follows: one case of cellular type, nine cases of mixed type, and eight cases of fibrous type lesions. Inflammatory cell infiltration and fibrous tissue proliferation were observed in the mixed type cases, which primarily comprised lymphocytes and a small number of plasmacytes. In the fibrous type cases, the inflammation was comparatively less, but the collagen deposition was significantly greater. The comparison between the pathologic characteristics in the NSIP and UIP patients is shown in Table 2.
Discussion
UIP and NSIP are the most common subtypes of IIP. In 2000 and 2002, the ATS and ERS respectively published reports detailing the diagnosis and treatment of IPF formed by consensus of experts worldwide. These reports described the most updated classification scheme (ATS/ERS classification) for IIP, including UIP, NSIP, and other subtypes (a total of seven subtypes). In 1994, Katzenstein et al. first described NSIP as interstitial pneumonia, which was not fit into other categories. In the new classification system, NSIP also excludes diseases with known causes, and presently includes three subtypes—cellular, fibrous, and mixed, which differ in their respective clinical manifestations, therapeutic options, and prognosis. The current ATS/ERS classification provides specific IIP categories, rather than employing general descriptions, which enables standardized diagnosis, treatment, and studies of IIP, and forms a foundation for domestic and international cooperation in research. However, the ATS/ERS classification requires clinical verification of its rationale and practicality. The ATS/ERS classification fails to address several questions concerning IIP. And the relationship between UIP and NSIP is still unclear. The disease course, treatment outcomes, and prognoses of the fibrous type of NSIP and UIP are quite similar, and techniques are unclear to distinguish them. The ideal pathologic, clinical and radiologic diagnostic criteria for UIP and NSIP require investigation and confirmation in additional cases. Large-scale studies in this field are presently lacking in China (5).
According to the present study, UIP occurred primarily in men over 50 years of age. More than 50% of the patients were over 60 years of age at the initial examination. This is in stark contrast to the mean onset age (48.2 years of age) in the NSIP patients. UIP occurred more frequently in men, while NSIP occurred more often in women. There were no significant differences in the clinical manifestations between the UIP and NSIP patients. Dry cough and dyspnea were main features in both groups, and inspiratory crackles were detected in most of the patients, which was most apparent at the base of both lungs. Clubbing was observed primarily in UIP patients. There were no significant differences between the UIP and NSIP patients in the respiratory function and laboratory findings. Although laboratory tests cannot diagnose UIP and NSIP, they can detect other diffuse lung diseases.
The chest radiographs were similarly ineffective in the diagnosis of UIP and NSIP; however, CT, especially HRCT, was quite useful diagnostically. On HRCT, the UIP cases showed patchy shadows in the basal regions of both lungs, and very few ground-glass opacities were present. Due to the severe fibrosis, traction bronchiectasis, bronchiolectasis, and subpleural honeycomb changes were observed in all UIP cases. NSIP mainly showed patchy and ground-glass opacities in both lungs. The honeycomb lesions were typically present in advanced cases of fibrous NSIP. HRCT was useful in the differential diagnosis of intractable cases. On HRCT, UIP was characterized by peripheral shadows and honeycomb changes. However, honeycomb changes were rare in NSIP (6), which only occurred late in the disease course (4,7).
The clinical and imaging characteristics were reportedly quite similar between the IIP subtypes; therefore, diagnosis relies on VATS or surgical lung biopsy findings (8). At low magnifications, NSIP showed phase consistency across the different fields, but the most distinctive characteristics were in the varying pathologic lesions, which comprised both acute and chronic features. The fibroblast foci in NSIP were small, and few were observed, with only 19.05% (4/21) of samples showing the foci. Honeycomb changes appeared later in the disease course in the fibrous type. Overall, it was difficult to distinguish mixed and fibrous NSIP, despite their different occurrence of fibroblast foci, honeycombing, myogelosis, diffuse collagen deposition, and alveolar restructuring. Therefore, comprehensive evaluation of these characteristics is necessary for the differential diagnosis (6,9,10).
In a previous study, the glucocorticoid response and prognosis differed between UIP and NSIP (11). We found that the UIP patients responded poorly to glucocorticoid therapy. Although a few patients experienced intermittent improvement, most received no significant therapeutic benefit even when cytotoxic drugs were added to the regimen. In contrast, the NSIP patients responded favorably to glucocorticoid therapy, and only patients with the fibrous type failed to respond; the glucocorticoid response rates were 38.89% and 76.19% in the two groups, respectively (P<0.01). On follow-up, six UIP patients died of respiratory failure 3 years after therapy, and the NSIP patients had an overall superior quality of life. These findings are consistent with those reported by Travis et al. (11), indicating that diagnosis based on the UIP and NSIP classifications have clinical significance. However, the NSIP pathology is clinically nonspecific; therefore, pathologic diagnosis mostly include both clinical and imaging data, namely a clinic-radiologic-pathologic diagnosis. This is a key point of IIP classification and diagnosis; a diagnosis made by clinical physicians, radiologists, or pathologists alone is likely to be biased. By investigating both the clinical history and laboratory examination data, conditions such as collagen vascular disease, tuberculous pulmonary fibrosis, occupational pulmonary fibrosis, eosinophilic pneumonia, and other interstitial lung disease can be excluded. Sampling errors may be a concern according to some studies; however, if the clinical manifestations and imaging features meet the criteria of UIP, then a lack of fibroblasts does not exclude the condition. According to the ATS/ERS classification, surgical lung biopsy (VATS or mini-incision surgical lung biopsy) is recommended in IIP patients without surgical contraindications in order to exclude other similar conditions and definitively diagnose IIP. It enables the implementation of effective therapies and prevents misdiagnosis and inappropriate treatment (12-17).
Conclusions
NSIP was difficult to be differentiated from UIP by general clinical manifestations, but HRCT can be helpful for this purpose. Definitive diagnosis depends on the results of surgical lung.
Acknowledgements
This work was supported by the funding of Shanghai Municipal Health Bureau Project (No. 054Y036).
Author contributions: Xia Li designed the overall study, carried out experiments, collected and analyzed data, and wrote the paper. Chang Chen performed the VATS and the open surgical lung biopsy procedures and collected data for histopathologic examinations. Jinfu Xu and Jinming Liu collected clinic data, and Xianghua Yi collected and analyzed histopathologic data. Xiwen Sun and Jingyun Shi collected and analyzed radiologic data.
Disclosure: The authors declare no conflict of interest.
References
- Travis WD, King TE, Bateman ED, et al. ATS/ERS consensus statement of idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;265:277-304. [PubMed]
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [PubMed]
- MacDonald SL, Rubens MB, Hansell DM, et al. Nonspecific interstitial pneumonia and usual interstitial pneumonia: comparative appearances at and diagnostic accuracy of thin-section CT. Radiology 2001;221:600-5. [PubMed]
- Honoré PM, Spapen H. Embracing western and Chinese research and clinical knowledge: We take up the translational gauntlet! J Transl Intern Med 2013;1:1-2.
- Kondoh Y, Taniguchi H, Yokoi T, et al. Cyclophosphamide and low-dose prednisolone in idiopathic pulmonary fibrosis and fibrosing nonspecific interstitial pneumonia. Eur Respir J 2005;25:528-33. [PubMed]
- Marten K, Milne D, Antoniou KM, et al. Non-specific interstitial pneumonia in cigarette smokers: a CT study. Eur Radiol 2009;19:1679-85. [PubMed]
- Popescu A, Săftoiu A. Can elastography replace fine needle aspiration? Endosc Ultrasound 2014;3:109-17. [PubMed]
- Riha RL, Duhig EE, Clarke BE, et al. Survival of patients with biopsy-proven usual interstitial pneumonia and nonspecific interstitial pneumonia. Eur Respir J 2002;19:1114-8. [PubMed]
- Travis WD, Hunninghake G, King TE Jr, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med 2008;177:1338-47. [PubMed]
- Travis WD, Matsui K, Moss J, et al. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 2000;24:19-33. [PubMed]
- Martinez FJ. Idiopathic interstitial pneumonias: usual interstitial pneumonia versus nonspecific interstitial pneumonia. Proc Am Thorac Soc 2006;3:81-95. [PubMed]
- Swigris JJ, Kuschner WG, Kelsey JL, et al. Idiopathic pulmonary fibrosis: challenges and opportunities for the clinician and investigator. Chest 2005;127:275-83. [PubMed]
- Fujita J, Ohtsuki Y, Yoshinouchi T, et al. Idiopathic non-specific interstitial pneumonia: as an “autoimmune interstitial pneumonia”. Respir Med 2005;99:234-40. [PubMed]
- Halkos ME, Gal AA, Kerendi F, et al. Role of thoracic surgeons in the diagnosis of idiopathic interstitial lung disease. Ann Thorac Surg 2005;79:2172-9. [PubMed]
- Monaghan H, Wells AU, Colby TV, et al. Prognostic implications of histologic patterns in multiple surgical lung biopsies from patients with idiopathic interstitial pneumonias. Chest 2004;125:522-6. [PubMed]
- Drent M, du Bois RM, Poletti V. Recent advances in the diagnosis and management of nonspecific interstitial pneumonia. Curr Opin Pulm Med 2003;9:411-7. [PubMed]


