
Comparison of the short-term outcomes of robot-assisted minimally invasive, video-assisted minimally invasive, and open esophagectomy
Introduction
Esophageal cancer (EC) ranks 7th in terms of incidence and 6th in mortality, of all cancer types, globally (1), and improved surgical protocols are required for its treatment. Surgical resection with radical lymphadenectomy remains the standard management for cancer patients with local resectable esophageal tumors (2). The Ivor Lewis esophagectomy (3) and McKeown esophagectomy (4) are commonly performed surgeries in the treatment of EC. Since their development, open esophagectomy (OE) has become a standard surgical procedure, particularly for patients experiencing local anatomical tumor invasion or adhesions from previous disease, radiotherapy, or surgery. Robot-assisted minimally invasive esophagectomy (RAMIE), first described in 2005 (5), can provide a full 3-dimensional vision of the operative field, which offers better exposure of the upper mediastinum for extensive paratracheal lymph node dissections (6,7). In recent years, video-assisted minimally invasive esophagectomy (VAMIE) has become the standard approach for EC surgery in a number of countries, which could decrease postoperative pain, result in fewer pulmonary complications and improve quality of life (8).
Recently, several studies have compared VAMIE and RAMIE. These studies have demonstrated a better quality of lymphadenectomy and comparable postoperative complications by RAMIE (9,10). However, OE was not included in these studies, and there was little discussion of the advantages and disadvantages of different surgical approaches.
The aim of this study was to compare the short-term outcomes of the OE, VAMIE, and RAMIE and to identify the clinical benefits of these surgical treatments in treating EC.
Methods
Patient selection
We retrospectively included all clinical data from patients who had undergone McKeown esophagectomy at Tianjin Medical University Cancer Institute and Hospital for Esophageal Cancer by the same four surgical teams from January 2016 to December 2018. Dr. Yu and Dr. Jiang performed OE, VAMIE, and RAMIE, while Dr. Zhao and Dr. Liu only performed OE and VAMIE. There were totally 558 cases underwent McKeown esophagectomy in our institute. But in the VAMIE group, we only included the cases that successfully underwent combined thoracoscopic and laparoscopic esophagectomy. In that case, 246 cases were only undergone thoracoscopic surgery. They were all excluded from the VAMIE group. As such, 312 cases qualified for this study (77 cases in the OE group, 144 cases in the VAMIE group, and 91 cases in the RAMIE). Patients who were suspected to have lymph node metastasis in the cervical area underwent the three-field lymph node dissection (N=31, 9.94%). And the rest patients were all underwent two-field lymph node dissection (N=281, 90.06%). All patients received gastrointestinal (GI) endoscopy, contrast-enhanced computed tomography scan of the chest and upper abdomen, and assessment of pulmonary function before the operation. Pathology results were evaluated according to eighth edition of TNM staging. Patients who received the neo-adjuvant therapy had been divided into two groups. One group received the neo-adjuvant chemo-radiotherapy. Patients received conformal radiotherapy with 40 Gy in 20 daily fractions, along with paclitaxel and Cisplatin once a week for 4 weeks. The other group received the neo-adjuvant chemotherapy. In this group, patients received the administration of paclitaxel with cisplatin, docetaxel with cisplatin, and 5-fluorouracil with cisplatin.
Approval for the study was obtained from the Ethics Committee of Tianjin Medical University Institute and Hospital (ID of the ethic approval: bc2019094). Written informed consent was obtained from each patient or his/her legal representative.
RAMIE surgical procedure
RAMIE was carried out using the robotic da Vinci Si System (Intuitive Surgical Inc., Sunnyvale, CA, USA). Patients that underwent OE were intubated with a left-side double-lumen tube, under general anesthesia. In contrast, patients who underwent VAMIE and RAMIE were intubated with single-lumen tube, favoring exposure of the upper mediastinal lymph nodes. For the thoracic protocol, patients were placed in the left lateral prone position. Trocars were designed as followed: robotic arm 1 was placed in the third intercostal space (ICS) on the middle axillary line; robotic arm 2 was placed in the ninth ICS between the posterior axillary line and scapular line; the camera port was placed in the sixth ICS along the posterior axillary line; and the assistant port was placed either in the fifth or seventh ICS along the anterior axillary line. The artificial pneumothorax was established by using CO2 at the pressure of 8–10 mmHg. For the abdominal protocol, patients were moved to the dorsal position. The trocar placement is shown in the Figure 1. A small incision was made for insertion of the gastric tube (3–4 cm wide). The gastric tube was then pulled up to the neck through the mediastinum. The anastomosis was formed by using a circular stapler.
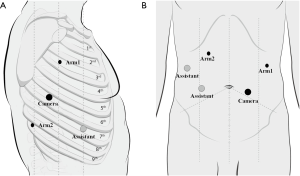
Data collection
We collected baseline data pertaining to gender, age, Charlson comorbidity index, tumor location, and histological types. Pathological outcomes, including pathology type, and the status of lymph node metastasis were also collected. All of the dissected lymph nodes were evaluated and grouped according to the lymph node station definition from the eighth edition of TNM staging (11). Postoperative complications were evaluated according to the Esophagectomy Complication Consensus Group complication definition. Pulmonary complications included were pneumonia, pneumothorax, respiratory failure requiring reintubation, acute respiratory distress syndrome, and tracheobronchial injury. Esophagogastric leak from anastomosis, staple line, or localized conduit necrosis were defined as full thickness GI defects involving esophagus, anastomosis, staple line, or conduit irrespective of presentation. Anastomosis leak was diagnosed by upper GI contrast or esophagography. Recurrent laryngeal nerve (RLN) injury was defined as vocal cord dysfunction, post-resection. Other complications included in this study were atrial fibrillation (AF), chylothorax, hemorrhage, and wound infection. Postoperative death was defined as death within 90 days after surgery.
Statistical analysis
Statistical analysis was performed using SPSS software version 22.0. Data were represented as the mean ± standard deviation (SD) for continuous variables or percentage (%) for categorical data. For continuous variables, Student’s t-test or Mann-Whitney U test was used, depending on normality of distribution; one-way analysis of variance (ANOVA) was used for three-way comparison of groups. For categorical data, the chi-square or Fishers exact test was applied; Kruskal-Wallis test was used for three-way comparison of groups. Statistical significance was set as a two-sided P value <0.05.
Results
A total of 312 patients met our cutoff criteria: 77 patients underwent OE, 144 underwent VAMIE, and 91 patients underwent RAMIE. The baseline clinical characteristics among three groups are shown in Table 1. There were no significant differences in demographic data between the three groups. The Charlson comorbidity indexes were similar among the three groups (P=0.592). There were more patients at the advanced stage in the OE group according to the cTNM stage (P=0.002) (Table S1). As a result, more than half of the patients received the neo-adjuvant therapies prior to surgical treatment in the OE group, compared with those two MIE groups (P=0.002). In the present study, the major histological type was squamous cell carcinoma. From the pathological results, we found a higher number of late stage patients in the OE group (P<0.001), resulting in more late stage patients in the OE group according to the pTNM stage (P=0.001) (Table 2).
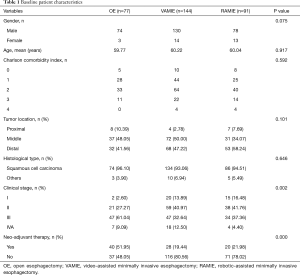
Full table
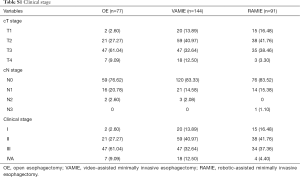
Full table
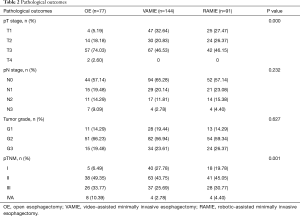
Full table
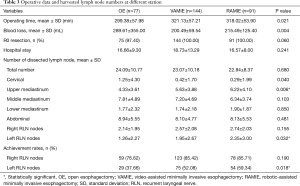
The surgical time in OE group was significantly shorter than the two MIE groups (P=0.021), with an approximate 20-minute difference. The blood loss volume in the OE group was significantly more compared with the two MIE groups (P=0.004). There were no significant differences for the hospital stay between the three groups (P=0.241) (Table 3).
Full table
There was no significant difference in the total dissected lymph node numbers between the three groups (OE: 24.09±10.77, VAMIE: 23.07±10.18, RAMIE: 22.84±8.37, P=0.680) (Table 3). Assessment of the dissected lymph nodes demonstrate that the number of upper mediastinal lymph nodes was significantly higher in the two MIE groups (OE: 4.33±3.61, VAMIE: 5.63±3.88, RAMIE: 6.22±4.10, P=0.006). The harvested right RLN nodes were comparable among the three groups. In contrast, we retrieved a greater number of nodes on the left RLN with the assistance of the robotic surgical system (OE: 1.26±2.27, VAMIE: 1.95±2.67, RAMIE: 2.35±3.00, P=0.032). The harvested nodes number at abdominal station was highly similar between the three groups. For improved quality control of the lymph node dissection in the RLN areas, we further analyzed the achievement rate of the RLN nodes’ dissection. We counted at least one lymph node per patient harvested from each RLN area as an effective dissection. Following this criterion, the achievement rates of the right RLN area were also similar in the three groups. We successfully dissected right RLN lymph nodes in more than 75% of patients. The achievement rate in the two MIE groups were significantly higher than the OE group in the left RLN area (OE: 37.66%, VAMIE: 52.08%, RAMIE: 59.34%, P=0.018). With the assistance of the robotic surgical system, the achievement rate increased in the RAMIE group.
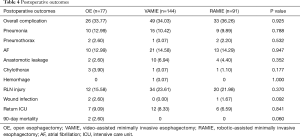
The overall postoperative complications rates were all above 30% (Table 4). The postoperative pneumonia rate decreased from 12.99% to 9.89% but there were no significant differences between three groups (P=0.788). The anastomotic leakage rates were no higher than 7% in each of the three groups (P=0.352). We started to us the enteral nutrition 24 hours after surgery. And we usually told the patients to start to drink water 7 days after the surgery. Then we told the patients to transit to normal food in the following 1 month. These kinds of nutrition support could be helpful for the control of the anastomotic leakage. Furthermore, the RLN injury rates were higher in the two MIE groups as a result of the higher achievement rates (P=0.370). The return intensive care unit (ICU) rate varied from 9.09% to 6.59% in each of the three surgical groups (P=0.841). The 90-day mortality rate was 2.60% in the OE group. Those two patients received neo-adjuvant therapies. The cause of the death was due to the severe pneumonia after the surgery. And there was no associated death in the other two groups. All other complications were comparable among the three groups.
Full table
Discussion
MIE has been in development for decades. It offers several advantages compared with the OE, including a decrease in post-operative pain and infection and improved recovery time. In recent years, usage of the da Vinci robotic surgical system appears to have improved recovery for patients undergoing surgical treatment for EC. Whilst many studies have compared the VAMIE and RAMIE systems, they have not, to our knowledge, compared these systems with OE. In our study, we compared all three groups, to provide a comprehensive overview of the outcomes of these different surgical interventions (12).
Lymph node status is an important prognostic parameter for EC patients and an independent predictor for survival (13). Lymph node dissection in EC can be classified as standard dissection, extended dissection, or three-field dissection (14). As there is insufficient evidence to claim that three-field is superior to two-field lymphadenectomy, the latter remains the prevalent procedure used in EC patients. In our study, 281 patients (90.06%) underwent two-field dissection. Previous studies compared the number of harvested lymph nodes between OE and VAMIE (15). The average total lymph node number in the OE group ranged from 13 to 25, without including the lymph nodes in the upper mediastinum. Similarly, the total number in the VAMIE group ranged from 15 to 27. However, in recent studies comparing VAMIE and RAMIE, the total lymph nodes number was greater. The average number was between 19 and 34 in the VAMIE group, and this increased to between 20 and 37 in the RAMIE group; however, this difference was not significant (16). In our study, there was no significant change in the number of lymph nodes harvested from the OE group (24.09), the VAMIE group (23.07), or the RAMIE group (22.84) (P=0.680). This may partly attribute to the higher percentages of late-stage patients and neo-adjuvant therapy in the OE group.
In recent years, the importance of the RLN lymphadenectomy, normally termed extended dissection, has been supported by many surgeons. Park et al. reported that they harvested more lymph nodes in upper mediastinum in the RAMIE group (RAMIE: 10.7, VAMIE: 6.3, P=0.032) (9). In Chao’s study, there were no significant changes in right RLN nodes dissection (RAMIE: 2.27, VAMIE: 2.77, P=0.226), but there was a significant increase in the harvested nodes from the left RLN in the RAMIE group (RAMIE: 5.32, VAMIE: 3.18, P=0.001) (7). Deng et al. reported that the number of lymph nodes from the right RLN area was higher in the RAMIE group (RAMIE: 2.1, VAMIE: 1.2, P=0.033) (10). As we describe in our study that the number of lymph nodes from the upper mediastinum in the OE group was significantly lower than those of the MIE groups (OE: 4.33, VAMIE: 5.63, RAMIE: 6.22, P=0.006). Furthermore, the number of lymph nodes from the left RLN area was also lower in the OE group (OE: 1.26, VAMIE: 1.95, RAMIE: 2.35, P=0.032). As reported in our study, we used the achievement rate as an important indicator for the quality control for lymphadenectomy in the RLN areas, and a higher percentage of achievement rates resulted in greater numbers of harvested lymph nodes. In the RAMIE group, the application of robotics offered better visualization and improved accuracy in dissection (17). Both the average number of lymph nodes and the achievement rates were highest in the bilateral RLN regions.
As we demonstrate in this study, VAMIE and RAMIE do not increase the overall perioperative complication rates compared with the OE group. RLN injury was the major postoperative complication. It has been reported that the RLN injury rate varies from 11.5% to 27.0% in OE groups, 14.3% to 28.8% in VAMIE groups, and from 9.5% to 21.6% in RAMIE groups (8,15). According to a meta-analysis by Yibulayin, there was no significant difference in RLN injury between the OE and VAMIE groups (15). In contrast, Jin et al. reported that VAMIE has a higher rate of RLN injury than RAMIE (18). In our study, achievement rates on the RLN nodes in the RAMIE group were higher than the other two groups, but the use of robotics did not significantly bring down the RLN injury rate. The pulmonary branches of the vagus nerve regulate many important pulmonary functions, such as the cough reflex, mucous production, and bronchus diameter (19). However, in our procedures we did not routinely reserve the pulmonary branches. This may explain why both the pulmonary complication rate and return ICU rate only decreased slightly according to different surgical approaches. Improved visualization of mediastinum structures, using robotic surgery, may afford better protection to the pulmonary branches, helping to limit pulmonary complications.
Another major complication, AF, was unchanged between the three groups in our study. Lohani et al. reported that the transthoracic approach is an independent risk factor for AF. It has been reported that 23.4% of patients with OE developed new-onset AF (20). In Day’s study, 31.4% patients suffered from AF following VAMIE. Older patients and patients who received neo-adjuvant chemoradiation showed a trend toward increased risk of AF (21). The use of minimally invasive robotic surgery does not appear to decrease the incidence of new-onset AF. Furthermore, surgical approaches do not affect the percentage of anastomotic leakage. These factors were unchanged between our three groups.
Neo-adjuvant therapy is an important therapeutic strategy for late stage EC patients, with approximately 27% of the patients in our study undergoing this therapy prior to surgery. This therapy appeared to have no impact on postoperative outcomes, regardless of the surgical approach used. Ma et al. confirm that the neo-adjuvant therapy does not appear to negatively impact the therapeutic outcome of MIE (22). Goel et al. also suggest that robot-assisted surgery was feasible and safe following neo-adjuvant therapy, with acceptable oncological outcomes and a shorter learning curve compared with the VAMIE (23). Further development of RAMIE could reduce the overall rate of complications caused by the neo-adjuvant therapy in the future.
It is important to note that the study presented here has several limitations. The first is the limited experience with robotic surgery prior to the study, which may affect the surgical outcomes when compared with the OE and VAMIE groups. Secondly, this was a retrospective study with small sample size, resulting in an imbalance of the TNM staging among the three groups. Finally, long term oncological outcomes are also needed in the evaluation of different surgical efficiency. Despite these limitations, our data appear to confirm those reported by a number of other studies, giving strength to the validity of our results.
Conclusions
Minimally invasive surgery is an established surgical approach and is becoming more common than open surgery for many procedures. RAMIE has further improved the advantages of lymphadenectomy, especially in the RLN lymph node areas. With the development of the surgical skills for MIE, it can reduce the complications caused by neo-adjuvant therapy used for the treatment of EC patients. However, large volume randomized controlled clinical trials may be needed in the future to verify safety, efficiency, and the impact on long term survival rate.
Acknowledgments
Funding: This work was supported by grant (81501994) from National Natural Science Foundation of China, and grant (320.2730.1886) from Wu Jieping Medical Foundation awarded to Lei Gong, grant (C1711) from Tianjin Medical University Cancer Institute and Hospital Clinical Trials Foundation awarded to Zhentao Yu, grant from (81501798) National Natural Science Foundation of China, and grant from (18JCYBJC95700) Natural Science Foundation of Tianjin awarded to Xi Zhang. Lei Gong: grant (HZB-20190528-11) from Bethune Charitable Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval for the study was obtained from the Ethics Committee of Tianjin Medical University Institute and Hospital (ID of the ethic approval: bc2019094). Written informed consent was obtained from each patient or his/her legal representative.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Gisbertz SS, Hagens ERC, Ruurda JP, et al. The evolution of surgical approach for esophageal cancer. Ann N Y Acad Sci 2018;1434:149-55. [Crossref] [PubMed]
- Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- McKeown KC. Total three-stage oesophagectomy for cancer of the oesophagus. Br J Surg 1976;63:259-62. [Crossref] [PubMed]
- Ruurda JP, Draaisma WA, van Hillegersberg R, et al. Robot-assisted endoscopic surgery: a four-year single-center experience. Dig Surg 2005;22:313-20. [Crossref] [PubMed]
- van der Horst S, Weijs TJ, Ruurda JP, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy for esophageal cancer in the upper mediastinum. J Thorac Dis 2017;9:S834-42. [Crossref] [PubMed]
- Chao YK, Hsieh MJ, Liu YH, et al. Lymph node evaluation in robot-assisted versus video-assisted thoracoscopic esophagectomy for esophageal squamous cell carcinoma: a propensity-matched analysis. World J Surg 2018;42:590-8. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Park S, Hwang Y, Lee HJ, et al. Comparison of robot-assisted esophagectomy and thoracoscopic esophagectomy in esophageal squamous cell carcinoma. J Thorac Dis 2016;8:2853-61. [Crossref] [PubMed]
- Deng HY, Huang WX, Li G, et al. Comparison of short-term outcomes between robot-assisted minimally invasive esophagectomy and video-assisted minimally invasive esophagectomy in treating middle thoracic esophageal cancer. Dis Esophagus 2018. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol 2017;12:36-42.
- Weksler B, Sullivan JL. Survival after esophagectomy: a propensity-matched study of different surgical approaches. Ann Thorac Surg 2017;104:1138-46. [Crossref] [PubMed]
- Hagens ERC, van Berge Henegouwen MI, Cuesta MA, et al. The extent of lymphadenectomy in esophageal resection for cancer should be standardized. J Thorac Dis 2017;9:S713-23. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Three-field lymph node dissection in esophageal cancer surgery. J Thorac Dis 2017;9:S731-40. [Crossref] [PubMed]
- Yibulayin W, Abulizi S, Lv H, et al. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol 2016;14:304. [Crossref] [PubMed]
- He H, Wu Q, Wang Z, et al. Short-term outcomes of robot-assisted minimally invasive esophagectomy for esophageal cancer: a propensity score matched analysis. J Cardiothorac Surg 2018;13:52. [Crossref] [PubMed]
- Takahashi C, Shridhar R, Huston J, et al. Esophagectomy from then to now. J Gastrointest Oncol 2018;9:903-9. [Crossref] [PubMed]
- Jin D, Yao L, Yu J, et al. Robotic-assisted minimally invasive esophagectomy versus the conventional minimally invasive one: A meta-analysis and systematic review. Int J Med Robot 2019;15:e1988. [Crossref] [PubMed]
- Weijs TJ, Ruurda JP, Luyer MDP, et al. New insights into the surgical anatomy of the esophagus. J Thorac Dis 2017;9:S675-80. [Crossref] [PubMed]
- Lohani KR, Nandipati KC, Rollins SE, et al. Transthoracic approach is associated with increased incidence of atrial fibrillation after esophageal resection. Surg Endosc 2015;29:2039-45. [Crossref] [PubMed]
- Day RW, Jaroszewski D, Chang YH, et al. Incidence and impact of postoperative atrial fibrillation after minimally invasive esophagectomy. Dis Esophagus 2016;29:583-8. [Crossref] [PubMed]
- Ma S, Yan T, Liu D, et al. Neoadjuvant chemotherapy followed by minimally invasive esophagectomy is safe and feasible for treatment of esophageal squamous cell carcinoma. Thorac Cancer 2018;9:310-5. [Crossref] [PubMed]
- Goel A, Shah SH, Selvakumar VPP, et al. Robot-assisted mckeown esophagectomy is feasible after neoadjuvant chemoradiation. Our Initial Experience. Indian J Surg 2018;80:24-9. [Crossref] [PubMed]


