
Early failure of mitral valve repair with anterior leaflet pericardial patch augmentation in rheumatic and radiation-induced valvulitis
Introduction
Rheumatic mitral valve disease remains an important cause of mitral regurgitation (MR), accounting for 7% to 15% of organic MR cases in large epidemiologic studies (1,2). Similarly, a history of radiation to the mediastinum, as is common in the treatment of several malignancies, is associated with future valve dysfunction in up to one-third of patients. Mitral valve repair (MVr) is favored over replacement for severe primary MR; this is due to a significant 30-day and mid to long-term mortality benefit, as well as a reduction in peri-operative complications (3,4). However, due to ongoing inflammation and leaflet infiltration, MVr in rheumatic or radiation-induced valvulitis may be associated with decreased durability and an increased risk of reoperation (5).
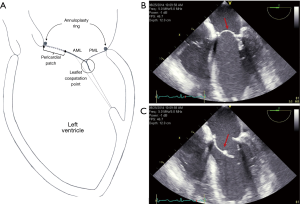
In order to improve outcomes of MVr in these populations, adjunctive reparative procedures have been introduced. One such technique involves the use of autologous or bovine pericardium to reconstruct or enlarge the anterior mitral leaflet (AML) and normalize its coaptation with the posterior leaflet, with further reinforcement from a supporting annuloplasty ring (Figure 1) (6). While small studies suggest excellent mid-term survival with this approach, moderate or greater recurrent MR and reoperation occurs in 11.2% and 4.6% of patients, respectively, with rheumatic MR. Poor outcome data exists in the literature regarding MVr techniques for radiation-induced valvulitis (6,7). Herein, we provide an in-depth clinical and imaging appraisal of two such cases, with a focused review on pathophysiology and management.
Methods and results
The technical and patient selection criteria and operative approach used in our institution’s prior surgical era for anterior leaflet augmentation MVr was previously described, and will be briefly reviewed (8). The anterior leaflet was carefully detached from the mitral annulus slightly past the posteromedial and anterolateral commissures, and then displaced towards the P2 scallop of the posterior leaflet. A portion of autologous or bovine pericardium measuring approximately 4 cm wide and 3 cm long, tailored to the size of the intercommisural diameter, was sutured to the mitral annulus and the detached basal portion of the anterior leaflet in an over-and-over fashion. A true-sized annuloplasty ring based on the length of the augmented anterior leaflet was used to stabilize the valvular apparatus (Video 1,2). Of note, the pericardial patch was not pretreated with glutaraldehyde to allow for flexible leaflet reconstruction and preservation of dynamic leaflet motion.
Patient one
A 69-year-old female with a history of rheumatic heart disease presented with combined severe rheumatic aortic stenosis and MR, and New York Heart Association (NYHA) functional class IV symptoms. The left ventricular systolic function [ejection fraction (LVEF) =66%] and size [end-diastolic diameter (LVEDD) =46 mm] were normal, and the pulmonary artery systolic pressure (PASP) measured 67 mmHg. On pre-operative echocardiography the mitral valve leaflet mid-body and tips were thickened with calcified and shortened chordae tendineae. An anterior leaflet augmentation MVr was performed utilizing a Peri-guard bovine pericardial patch (Baxter, Deerfield, IL, USA) and a 35 mm St. Jude Tailor annuloplasty ring (St. Jude Medical, St. Paul, MN, USA), in addition to bioprosthetic aortic valve replacement with a 23 mm Medtronic Mosaic valve (Medtronic Inc., Minneapolis, MN, USA), MAZE procedure, and left atrial appendage ligation. The patient had an uneventful post-operative course and was discharged on day 4.
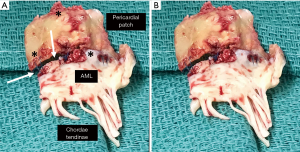
At 4 years post-MVr she developed progressive dyspnea on exertion, recurrent atrial fibrillation, and NYHA class III symptoms. Echocardiography revealed severe recurrent MR secondary to extensive patch and native anterior leaflet fibrocalcific degeneration with partial patch flail (Video 3,4). The LVEF, LVEDD, and PASP measured 64%, 49 mm, and 61 mmHg, respectively. A reoperative mitral valve replacement was performed utilizing a 29 mm Carpentier-Edwards Magna Ease bioprosthesis (Edwards Lifesciences, Irving, CA, USA). Gross pathology revealed mitral leaflet and pericardial patch sclerosis, focal calcifications, and granulomatous deposition (Figure 2). Post-operatively the patient was treated for right ventricular failure, which required an extended intensive care unit length of stay and inotropic support, with good eventual recovery. She was discharged home on day 15.
Patient two
A 53-year-old female with a history of Hodgkin’s lymphoma and mediastinal radiation presented with severe MR, NYHA functional class II heart failure symptoms, normal left ventricular systolic function (LVEF =65%) and size (LVEDD =45 mm), and a PASP of 50 mmHg. On pre-operative echocardiography the mitral valve was diffusely thickened and calcified, with restricted motion of the leaflet tips. An anterior leaflet augmentation MVr was performed utilizing an autologous pericardial patch and a 31 mm St. Jude Tailor annuloplasty ring (St. Jude Medical, St. Paul, MN, USA). The patient had an uneventful post-operative course and was discharged on day 3.
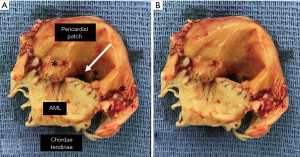
At 4 years post-MVr she developed progressive dyspnea on exertion and NYHA class III symptoms. Echocardiography revealed severe recurrent MR secondary to patch calcification, retraction, and central dehiscence, as well as moderate-to-severe secondary/functional tricuspid regurgitation. The LVEF, LVEDD, and PASP measured 63%, 47 mm, and 66 mmHg, respectively. A combined reoperative mitral valve replacement and tricuspid valve repair was performed, utilizing a 31 mm St. Jude Masters mechanical mitral valve prosthesis (St. Jude Medical, St. Paul, MN, USA) and a 26 mm Carpentier-Edwards tricuspid valve annuloplasty ring (Edwards Lifesciences, Irving, CA, USA). Gross pathology revealed diffuse dystrophic calcification with focal areas of hemorrhage (Figure 3). The patient had an uneventful post-operative course and was discharged on day 7.
Conclusions
The feasibility of MVr for rheumatic or radiation-induced mitral valve disease and MR is highly dependent upon several factors including: (I) anterior leaflet pliability; (II) severity and extent of subvalvular apparatus calcification; and, (III) the ability to address pseudoprolapse of the AML, which often occurs in the setting of a severely diseased posterior leaflet with chordal retraction and leaflet tethering (9,10). In addition to leaflet and subvalvular debridement and mobilization, annuloplasty rings are pivotal to remodel and stabilize the mitral annulus. A minimum ring size of 30 mm in females and 32 mm in males is recommended to avoid iatrogenic mitral stenosis in the setting of instric leaflet abnormality, which may require leaflet pericardial patch enlargement to increase the surface area and height, as in the present cases (11).
Pericardial tissue is mainly composed of cross-linked collagen fibers and is used in reconstructive cardiac operations, including complex MVr, due to its elastic and conformative properties (12,13). Autologous and bovine pericardium are nonthrombogenic with a very high threshold for infection; autologous patches are also nonantigenic (13,14). Pretreatment with glutaraldehyde may be performed to stiffen and improve handling of the pericardium, however, there is a risk of patch calcification and retraction post-implantation (15). An alternative to bovine or autologous pericardium in valvular reconstruction is decellularized porcine small intestine submucosa (CorMatrix, CorMatrix Cardiovascular Inc., Roswell, GA, USA). Despite its ability to stimulate cell differentiation and host cell ingrowth, there is a concern regarding immune-mediated patch degeneration and a rate of recurrent severe MR as high as 32% after CorMatrix leaflet augmentation (15,16).
Failure of anterior leaflet pericardial patch augmentation MVr in patient one was the consequence of the progressive exudative inflammatory processes of her rheumatic disease (9). A complex immunologic reaction triggers the deposition of granulomatous Aschoff bodies and fibrin on the pericardial patch and mitral valve leaflets, in particular at the leaflet tips and chordal apparatus, as well as osteoblast upregulation and neoangiogenesis (18-20). In patient two with radiation-induced valvulitis, osteogenic and fibrogenic growth factors induce mitral leaflet fibrosis and calcification, similar to bone formation; converse to rheumatic mitral valves, there are no inflammatory or vascular changes (21-23). Both pathologies resulted in destruction of the pericardial patch-AML continuity (suture line), with patch dehiscence and partial flail.
While pericardial leaflet augmentation MVr for rheumatic disease appears to have a lower rate of reoperation for recurrent MR when compared with conventional MVr at early 3- to 5-year follow-up (4.6% vs. 8% to 13%), it is similar to what patients with organic/degenerative MR experience at a much longer surveillance of 20 years (7,24). This is important as data from the Society of Thoracic Surgeons National Database shows that reoperative mitral valve surgery has an operative mortality of 6.6%, with new onset renal failure and stroke occurring in 5.6% and 2.4%, respectively (25). Post-operative right ventricular failure, as seen in the case of patient one, occurs in up to a quarter of cardiac operations and is associated with an in-hospital mortality of 35% to 50% (26,27). Thus, stratification of patients with rheumatic or radiation-induced valvular heart disease at risk for progressive sequelae and early reoperation post-MVr, both with pericardial leaflet augmentation or conventional techniques, is warranted. Carpentier and colleagues reported age, calcium metabolism, and chronic kidney disease as the most important patient-related factors, and inflammatory response (i.e., extent of valvular apparatus involvement) and tissue components and preservation as the valve-related factors associated with patch MVr failure for rheumatic MR (6,28,29). While no such data exists for radiation valvulitis, a cutoff of 33 Gy for whole heart radiation dose or 30 Gy to either the left atrium or left ventricle identifies patients at higher risk of valvular disease (30,31). In the above scenarios, the risk versus benefit discussion of MVr and chordal-sparing mitral valve replacement should be at the center of a multi-disciplinary Heart Valve Team approach.
In conclusion, MVr utilizing an annuloplasty ring in conjunction with AML pericardial patch augmentation is feasible in rheumatic and radiation-induced mitral valve disease and MR. However, careful patient selection for MVr versus mitral valve replacement is paramount as rheumatic and radiation valvulitis are ongoing, smoldering processes with incremental risk for MVr failure and reoperation in this higher risk population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christos G. Mihos) for the series “Novel Concepts in Cardiopulmonary and Structural Heart Disease” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.01.20). The series “Novel Concepts in Cardiopulmonary and Structural Heart Disease” was commissioned by the editorial office without any funding or sponsorship. CGM served as the unpaid Guest Editor of the series and serves as an unpaid editorial member of Journal of Thoracic Disease from Jan 2019 to Dec 2020. FN serves as an unpaid editorial member of Journal of Thoracic Disease from Aug 2019 to Jul 2021. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dziadzko V, Dziadzko M, Medina-Inojosa JR, et al. Causes and mechanisms of isolated mitral regurgitation in the community: clinical context and outcome. Eur Heart J 2019;40:2194-202. [Crossref] [PubMed]
- Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol 2014;30:962-70. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Wang Z, Zhou C, Gu H, et al. Mitral valve repair versus replacement in patients with rheumatic heart disease. J Heart Valve Dis 2013;22:333-9. [PubMed]
- Chauvaud S, Jebara V, Chachques JC, et al. Valve extension with glutaraldehyde-preserved autologous pericardium. Results in mitral valve repair. J Thorac Cardiovasc Surg 1991;102:171-7; discussion 177-8. [Crossref] [PubMed]
- Mihos CG, Pineda AM, Capoulade R, et al. A systematic review of mitral valve repair with autologous pericardial leaflet augmentation for rheumatic mitral regurgitation. Ann Thorac Surg 2016;102:1400-5. [Crossref] [PubMed]
- Mihos CG, Pineda AM, Horvath SA, et al. Anterior mitral leaflet augmentation for ischemic mitral regurgitation performed via a right thoracotomy approach. Innovations (Phila) 2016;11:298-300. [Crossref] [PubMed]
- Roberts S, Kosanke S, Terrence Dunn S, et al. Pathogenic mechanisms in rheumatic carditis: focus on valvular endothelium. J Infect Dis 2001;183:507-11. [Crossref] [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [Crossref] [PubMed]
- Carpentier A, Adams DH, Filsoufi F. Carpentier’s reconstructive valve surgery: from valve analysis to valve reconstruction. Maryland Heights: Saunders; 2010.
- Goissis G, Giglioti Ade F, Braile DM. Preparation and characterization of an acellular bovine pericardium intended for manufacture of valve bioprostheses. Artif Organs 2011;35:484-9. [Crossref] [PubMed]
- Lam MT, Wu JC. Biomaterial applications in cardiovascular tissue repair and regeneration. Expert Rev Cardiovasc Ther 2012;10:1039-49. [Crossref] [PubMed]
- ASM International. Materials and coatings for medical devices: cardiovascular. Novelty: ASM International, 2009:12-8.
- Vincentelli A, Zegdi R, Prat A, et al. Mechanical modifications to human pericardium after a brief immersion in 0.625% glutaraldehyde. J Heart Valve Dis 1998;7:24-9. [PubMed]
- Kelley TM Jr, Kashem M, Wang H, et al. Anterior leaflet augmentation with CorMatrix porcine extracellular matrix in twenty-five patients: unexpected patch failures and histologic analysis. Ann Thorac Surg 2017;103:114-20. [Crossref] [PubMed]
- Mosala Nezhad Z, Poncelet A, de Kerchove L, et al. Small intestinal submucosa extracellular matrix (CorMatrix®) in cardiovascular surgery: a systematic review. Interact Cardiovasc Thorac Surg 2016;22:839-50. [Crossref] [PubMed]
- Guilherme L, Cury P, Demarchi LM, et al. Rheumatic heart disease: proinflammatory cytokines play a role in the progression and maintenance of valvular lesions. Am J Pathol 2004;165:1583-91. [Crossref] [PubMed]
- Marcus RH, Sareli P, Pocock WA, et al. The spectrum of severe rheumatic mitral valve disease in a developing country. Correlations among clinical presentation, surgical pathologic findings, and hemodynamic sequelae. Ann Intern Med 1994;120:177-83. [Crossref] [PubMed]
- Artola RT, Mihos CG, Santana O. Immunology of mitral valve stenosis. Int J Interferon Cytokine Mediator Res 2011;3:1-8.
- Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol 1996;27:766-73. [Crossref] [PubMed]
- Brosius FC 3rd, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med 1981;70:519-30. [Crossref] [PubMed]
- Gujral DM, Lloyd G, Bhattacharyya S. Radiation-induced valvular heart disease. Heart 2016;102:269-76. [Crossref] [PubMed]
- David TE, David CM, Tsang W, et al. Long-term results of mitral valve repair for regurgitation due to leaflet prolapse. J Am Coll Cardiol 2019;74:1044-53. [Crossref] [PubMed]
- Kilic A, Acker MA, Gleason TG, et al. Clinical outcomes of mitral valve reoperations in the United States: an analysis of The Society of Thoracic Surgeons National Database. Ann Thorac Surg 2019;107:754-9. [Crossref] [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. [Crossref] [PubMed]
- Denault AY, Pearl RG, Michler RE, et al. Tezosentan and right ventricular failure in patients with pulmonary hypertension undergoing cardiac surgery: the TACTICS trial. J Cardiothorac Vasc Anesth 2013;27:1212-7. [Crossref] [PubMed]
- Carpentier A, Dubost C, Lane E, et al. Continuing improvements in valvular bioprostheses. J Thorac Cardiovasc Surg 1982;83:27-42. [Crossref] [PubMed]
- Carpentier A, Nashef A, Carpentier S, et al. Techniques for prevention of calcification of valvular bioprostheses. Circulation 1984;70:I165-8. [PubMed]
- Cella L, Liuzzi R, Conson M, et al. Dosimetric predictors of asymptomatic heart valvular dysfunction following mediastinal irradiation for Hodgkin's lymphoma. Radiother Oncol 2011;101:316-21. [Crossref] [PubMed]
- Cella L, Liuzzi R, Conson M, et al. Multivariate normal tissue complication probability modeling of heart valve dysfunction in Hodgkin lymphoma survivors. Int J Radiat Oncol Biol Phys 2013;87:304-10. [Crossref] [PubMed]


