
Use of imaging studies to predict postoperative recurrences of primary spontaneous pneumothorax
Introduction
Primary spontaneous pneumothorax (PSP) is a relatively common event in young and predominantly male adults (men: 18–28 per 100,000; women: 1–9.8 per 100,000) (1-3). Most of the patients are healthy, showing no underlying lung disease. Emergency situations are quite rare, but the potential for recurrence is troublesome. At 1 year, the rate of recurrence is ~30% (4,5). Subpleural blebs or bullae are the primary cause of PSP, and bullectomy with pleural procedure is the most curative means of treatment (6).
Since 1990s, video-assisted thoracoscopic surgery (VATS) has assumed a major position in the field of thoracic surgery (7), sparing patients the greater trauma of thoracotomy. Although VATS is now considered the first surgical option in PSP, postoperative recurrences are relatively frequent, perhaps due to the following: (I) missed ruptured bulla or bleb at time of surgery, (II) neobulla formation at staple lines, and (III) other sites of bulla formation. Currently, neobulla formation at staple lines is known as the major cause of postoperative recurrence (8,9).
Although a number of studies have tried to address the risk factors for postoperative recurrence, the fundamental pathology is unclear. However, based on a recent study implicating tension at staple lines (10,11), residual apical space is the narrowest in the pleural cavity. It is not easy to obliterate pleural residual cavity after bullectomy because remained apical resection area with staples showed rigidity. We have presumed that there is a chest radiographic evidence of tension at the staple line which may induce postoperative recurrence.
Methods
The Institutional Review Board of Bucheon St. Mary’s Hospital granted approval for this retrospective review (HC18RESI0108). All pertinent data were obtained from the electronic medical record (EMR) system, providing archives for the period of January 2013 to September 2016. Patients with underlying lung diseases (i.e., emphysema or tuberculosis), those undergoing thoracotomy, or any who lacked required medical records were excluded.
A total of 154 procedures were deemed acceptable for review, including seven patients subjected to bilateral bullectomies. VATS procedures were performed exclusively, some by uniportal access (53/144, 36.8%). Prior to each procedure, high-resolution computed tomography (HRCT) was obtained. Operative indications were recurrent pneumothorax, history of prior contralateral pneumothorax, total lung collapse, persistent air leakage (>3–4 days) after closed-tube thoracostomy, and visible bullae on HRCT.
In terms of surgical technique, standard three-port VATS or uniportal access was used. In three-port VATS, we used 5 mm port for thoracoscopy, another 5 mm port for instruments and 11.5 mm port for stapler. In uniportal procedure, 1.5 cm skin incision was made on the 5th intercostal space at the mid-axillary line or the wound of previous chest tube was used. Eleven-point five mm port was placed and 5 mm thoracoscopy was advanced through the port then the port was taken out along the thoracoscopy. One-zero nylon suture was passed from outside chest wall into the thoracic cavity through the 3rd intercostal space and the needle was grasped using endoscopic needle holder through the incisional space in the thorax. Stay suture was placed at the bulla and the needle was taken out through the incision for lung lifting. Bullectomy was performed using endostapler through the same incision (12). Bullectomy with abrasion of apical parietal and visceral pleura is stipulated at our hospital. The staple lines were covered with polyglycolic acid (PGA) sheets (NEOVEIL; Gunze, Ayabe, Japan) and fibrin glue in all of the cases. Division of the ligament was not performed. Apical chest tubes were then placed.
Intravenous patient-controlled analgesia (PCA) using fentanyl was conducted in all of the patients and the chest tube was removed in the absence of air leakage regardless of the presence of residual pleural cavity and diaphragmatic tenting.
Prior to discharge, all patients were monitored through daily chest radiography. Discharge generally took place the day after chest tube removal. Chest radiography was also obtained 7–8 days after discharge at the outpatient clinic. Postoperative recurrence was defined by chest radiography or computed tomography (CT) findings. Even minimal development of pleural air space (relative to previous imaging studies) qualified as postoperative recurrence regardless of symptoms according to the definition of pneumothorax.
The definition of diaphragmatic tenting is diaphragmatic elevation with subsegmental atelectasis in lower lobe on chest radiography for obliteration of residual pleural cavity after operation (Figures 1,2, red circle).
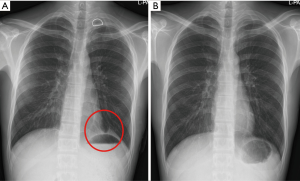
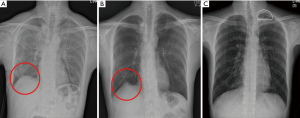
Postoperative diaphragmatic tenting or residual pleural cavity means the presence of these findings on chest radiography during postoperative period and remained (prolonged) diaphragmatic tenting or residual pleural cavity means the presence of these findings on chest radiography a week after discharge.
Two reviewers re-evaluated chest radiography of the patients independently. If the opinion was different between two reviewers. The consensus was achieved after discussion.
Statistical analysis
Medical records and imaging studies were reviewed and a telephone surgery was conducted regarding postoperative recurrence.
Data were expressed accordingly as median (minimum–maximum) or frequencies and percentages. Chi-square test was applied to categorical variables, using Mann-Whitney U test for continuous variables. Cox proportional hazard model was carried out to identify risk factors for postoperative recurrence, and multivariate analysis was thereafter performed. Standard software [SPSS v18; SPSS Inc. (IBM), Chicago, IL, USA] was engaged for all computations, setting statistical significance at P<0.05.
Results
In this study, 154 procedures involving 147 patients were reviewed. The patient characteristics were demonstrated in Table 1. The majority of the cases were male (144/154, 93.5%). The median age at the time of operation was 19 (range, 15–39) years. The median height was 175 (range, 149–195) cm and the median body weight was 58.5 (range, 40–82) kg. The median body mass index (BMI) was 19 (range, 15.26–26.47) kg/m2. Thirty-six cases were smoker (23.4%). Seventy-nine cases were left-sided pneumothorax (51.3%) whereas 61 cases showed right sided pneumothorax (39.6%). Closed thoracostomies were performed in 98 cases before operation (63.6%). Uniportal bullectomy was conducted in 57 cases (37.0%).
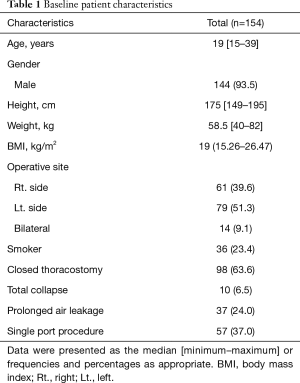
Full table
On initial chest radiography, total lung collapse was evident in 10 cases (6.5%), whereas 37 cases (24.0%) showed prolonged air leakage before surgery. Median operative time was 35 (range, 15–120) min. Although bilateral bullectomies required more time, there were no intraoperative complications. The specimen size was measured in all of patients. Transverse diameter (median, 7.5 cm; range, 1.4–14.0 cm); width (median, 2.1 cm; range, 1.0–4.0 cm); and height (median, 1.8 cm; range, 0.7–3.2 cm) were recorded.
Median duration of chest tube placement was 2 [0–10] days. Median postoperative hospital stay was 3 [2–10] days. Immediate postoperative air leakage was showed in 18 cases.
All patients were monitored postoperatively through daily chest radiography. Diaphragmatic tenting (50.6%) and residual pleural cavity (66.2%) were identified during the postoperative period (Figure 1). Patients were discharged at the day after chest tube removal and checked chest radiography 7–8 days later at the outpatient clinic. At this time, prolonged diaphragmatic tenting was noted in 38 cases (24.7%), and 52 (33.8%) cases showed prolonged residual pleural cavity (Figure 2). The maximal vertical diameter of apical residual space (median, 0 mm; range, 0–32 mm) was measured on chest radiography. In 76 cases (49.4%), chest radiographic findings were entirely normal (Table 2).
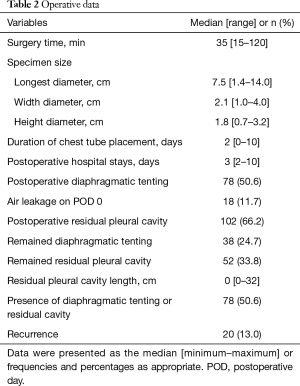
Full table
The median follow-up period was 52 (range, 28–70) months. Postoperative recurrence was developed in 20 patients (13.0%), re-operation was indicated for patients with second episode of recurrence after operation. Total 10 patients showed minimal pneumothorax after operation and oxygen was administered to 5 patients with symptoms. Other five asymptomatic patients showed only apical small pneumothorax compared with previous chest radiography without any treatment. In these patients, 3 patients showed second episode of pneumothorax after operation but they refused re-operation during follow-up period. In other 10 patients with recurrence, 2 patients required closed thoracostomy. Eight patients underwent re-operations. One patient showed prolonged air leakage after bullectomy, attributed to an initially missed bulla. Re-operation was carried out a week after first operation and tiny ruptured bulla was identified. In other 7 patients, bulla at the staple line was identified in 5 patients and 2 patients had bulla at the other sites. HRCT was conducted in 16 patients with postoperative recurrence. Visible bulla was identified in 6 patients on HRCT.
The patients were divided to two groups according to recurrence (Table 3). There were no significant differences between two groups in age, height, gender and BMI. Pathologic specimen size was compared between two groups but there were no differences. Current smokers were significantly higher in non-recurrence group (P=0.004). The proportion of uniportal surgery was no significant difference between two groups. Median postoperative hospital stay of recurrence and non-recurrence group was 3.4 and 3.2 days, respectively (P=0.415). Immediate postoperative air leakage was showed 5 patients in recurrence group and 13 patients in non-recurrence group (P=0.062). In recurrence group, postoperative diaphragmatic tenting on chest radiography was developed in 10 patients (50.0%) and 68 patients (50.7%) showed in non-recurrence group without difference. There was no difference in postoperative residual pleural cavity between two groups (P=0.454). However, chest radiographies were carried out a week after discharge. Remained diaphragmatic tenting was showed in 9 patients (45.0%) in recurrence group and 29 patients (21.6%) in non-recurrence group with significant difference (P=0.047). Although the proportion of remained residual pleural cavity was no significant difference between two groups, median apical residual space diameter was significantly higher in recurrence group (P=0.048).

Full table
In univariate analysis, smoking is not associated with recurrence (P=0.115). Factors of significance in recurrences of PSP were remained diaphragmatic tenting (P=0.026) and length of apical residual space (P=0.024) on chest radiography. In multivariate analysis (Table 4), both similarly showed significance (P=0.020 and P=0.018, respectively).

Full table
Discussion
Pneumothorax is an accumulation of air in the pleural space that may induce lung collapse. Affected patients present respiratory symptoms including cough, chest pain, and dyspnea. In most instances, there is a bulla formation, usually located at the visceral pleura of apical lung. Typically, patients with PSP are young and healthy, showing no underlying disease so emergent situation is rare. However, the risk of subsequent recurrence may be a lingering source of stress.
Resection of predisposing localized lesion is the most effective treatment for PSP. Since VATS has been introduced in 1990s, VATS has gradually gained in popularity. It is a simple and safe approach in this setting, marked by automatic endoscopic (rather than manual) suturing devices. However, recurrence rates after VATS have exceeded those of open thoracotomy (13,14). The various pleural procedures have been advised to decrease recurrence rate after operation. The additional pleural procedures included pleurectomy, pleural abrasion and pleurodesis. It is clear that bullectomy with pleural procedures showed lower recurrence rate than bullectomy alone (15-19).
As many thoracic surgeons will agree that younger age is a risk factor for postoperative recurrence after operation in PSP (20,21) and pneumothorax may be associated with growth (22). Other risk factors are still controversy. Given the simplicity of diagnosis and treatment, chest radiography and preoperative CT is enough for both detection and strategic treatment in PSP. We could not find clinical or pathological characteristics in patients with PSP compared with other people without PSP and there are no discernible differences to predict for recurrences. Although neo-bulla formation (at staple lines or elsewhere) is the fundamental issue, the mechanisms remain unclear.
Tsuboshima et al. have reported a correlation between new postoperative bullae and resected lung weight (10). After VATS bullectomy, residual lung tissue must compensate for the residual pleural cavity. Tension at the staple line may consequently become inordinate, due to the rigidity of automatic suturing, and eventually leads to over-inflation of remained lung and diaphragmatic elevation for upward direction of remained lung. Choi et al. have also maintained that lung volume is a risk factor for postoperative recurrence (11), and another study showed that apical residual space after bullectomy is associated with recurrences, citing early pleural symphysis failure as the basis (23). After apical bullectomy in spontaneous pneumothorax, remained lung has to be lifted upward direction to obliterate the space. Diaphragmatic tenting and residual pleural cavity were common findings on the chest radiography. In our study, about half of the patients showed diaphragmatic tenting on postoperative chest radiography, and in more than half, residual pleural cavity persisted during postoperative period.
One particular patient with recurrent PSP was pivotal in triggering the present study. Eight months after VATS bullectomy, chest pain of 2-day’s duration was claimed, with no chest radiographic abnormalities at that time of visit and 3 months later, recurrent pneumothorax was diagnosed with same symptoms. We reviewed the previous serial imaging studies of the patient. There was newly identifiable diaphragmatic tenting (without pneumothorax) on the chest radiography before 3 months, and we presumed that pneumothorax was actually presented 8 months after operation and spontaneous regression was achieved at the time of visit. It led to diaphragmatic tenting for obliteration of residual space. Hence, we speculated that presence of diaphragmatic tenting on chest radiography may be related to recurrence or the persistence of diaphragmatic tenting and residual pleural cavity after surgery may be associated with recurrence.
After VATS bullectomy in our patients, diaphragmatic tenting and residual pleural cavity are common findings on chest radiography, although they gradually disappear through expansion or shifting of residual lung tissue within a months after operation. Immediate postoperative features on chest radiography did not achieve statistical significance in our analysis. Once discharged, however, about one-quarter of our patients showed continued diaphragmatic tenting, and one-third harbored residual pleural cavity. We thought that constant tension at staple lines may therefore be needed to reduce residual pleural space, furthermore residual pleural space is the narrowest and cone-shaped in the apical thorax so obliteration of this space is not easy. We speculated that this mechanism induces over expansion of remained upper lobe, neo-bulla formation and increases the risk of recurrence after operation.
There are certain limitations to the present study. The number of patients was small, and our review was retrospective in nature. The frequency of postoperative recurrences was also relatively higher than other studies. However, we reviewed every chest radiographies of each patient several times, and five patients with marginal evidence of recurrence may have unduly inflated the rate. Although the patients herein were operated upon by two surgeons using differing techniques, no statistical differences were evident.
In conclusion, diaphragmatic tenting and residual pleural cavity that persist on chest radiography after VATS bullectomy may signal potential recurrence of PSP. A larger-scale, randomized, and prospective study, with more definitive follow-up by CT, may be required for corroboration.
Acknowledgments
This manuscript has been edited by native English-speaking experts of BioMed-Proofreading.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2019.11.46). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from all the patients. The Institutional Review Board of Bucheon St. Mary’s Hospital granted approval for this retrospective review (HC18RESI0108).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Melton LJ 3rd, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis 1979;120:1379-82. [PubMed]
- Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. [Crossref] [PubMed]
- Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666-71. [Crossref] [PubMed]
- Noppen M, Alexander P, Driesen P, et al. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax: a multicenter, prospective, randomized pilot study. Am J Respir Crit Care Med 2002;165:1240-4. [Crossref] [PubMed]
- Ayed AK, Chandrasekaran C, Sukumar M. Aspiration versus tube drainage in primary spontaneous pneumothorax: a randomised study. Eur Respir J 2006;27:477-82. [Crossref] [PubMed]
- Herrmann D, Klapdor B, Ewig S, et al. Initial management of primary spontaneous pneumothorax with video-assisted thoracoscopic surgery: a 10-year experience. Eur J Cardiothorac Surg 2016;49:854-9. [Crossref] [PubMed]
- Waller DA, Forty J, Morritt GN. Video-assisted thoracoscopic surgery versus thoracotomy for spontaneous pneumothorax. Ann Thorac Surg 1994;58:372-6; discussion 376-7. [Crossref] [PubMed]
- Muramatsu T, Ohmori K, Shimamura M, et al. Staple line reinforcement with fleece-coated fibrin glue (TachoComb) after thoracoscopic bullectomy for the treatment of spontaneous pneumothorax. Surg Today 2007;37:745-9. [Crossref] [PubMed]
- Cho S, Jheon S, Kim DK, et al. Results of repeated video-assisted thoracic surgery for recurrent pneumothorax after primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2018;53:857-61. [Crossref] [PubMed]
- Tsuboshima K, Nagata M, Wakahara T, et al. Association between postoperative bulla neogenesis at the staple line and resected lung weight for primary spontaneous pneumothorax: a retrospective study using the inverse-probability of treatment weighted method in patients grouped according to age. J Thorac Dis 2016;8:3676-81. [Crossref] [PubMed]
- Choi SY, Kim DY, Suh JH, et al. New bullae formation in the staple line increases the risk of recurrent pneumothorax following video-assisted thoracoscopic surgery bullectomy for primary spontaneous pneumothorax. J Thorac Dis 2018;10:4287-92. [Crossref] [PubMed]
- Jeon HW, Kim YD. Does 11.5 mm guided single port surgery has clinical advantage than multi-port thoracoscopic surgery in spontaneous pneumothorax? J Thorac Dis 2016;8:2924-30. [Crossref] [PubMed]
- Chan P, Clarke P, Daniel FJ, et al. Efficacy study of video-assisted thoracoscopic surgery pleurodesis for spontaneous pneumothorax. Ann Thorac Surg 2001;71:452-4. [Crossref] [PubMed]
- Delpy JP, Pagès PB, Mordant P, et al. Surgical management of spontaneous pneumothorax: are there any prognostic factors influencing postoperative complications? Eur J Cardiothorac Surg 2016;49:862-7. [Crossref] [PubMed]
- Ling ZG, Wu YB, Ming MY, et al. The effect of pleural abrasion on the treatment of primary spontaneous pneumothorax: a systematic review of randomized controlled trials. PLoS One 2015;10:e0127857. [Crossref] [PubMed]
- Lee S, Kim HR, Cho S, et al. Staple line coverage after bullectomy for primary spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014;98:2005-11. [Crossref] [PubMed]
- Hatz RA, Kaps MF, Meimarakis G, et al. Long-term results after video-assisted thoracoscopic surgery for first-time and recurrent spontaneous pneumothorax. Ann Thorac Surg 2000;70:253-7. [Crossref] [PubMed]
- Horio H, Nomori H, Kobayashi R, et al. Impact of additional pleurodesis in video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax. Surg Endosc 2002;16:630-4. [Crossref] [PubMed]
- Cardillo G, Bintcliffe OJ, Carleo F, et al. Primary spontaneous pneumothorax: a cohort study of VATS with talc poudrage. Thorax 2016;71:847-53. [Crossref] [PubMed]
- Noh D, Lee S, Haam SJ, et al. Recurrence of primary spontaneous pneumothorax in young adults and children. Interact Cardiovasc Thorac Surg 2015;21:195-9. [Crossref] [PubMed]
- Jeon HW, Kim YD, Kye YK, et al. Air leakage on the postoperative day: powerful factor of postoperative recurrence after thoracoscopic bullectomy. J Thorac Dis 2016;8:93-7. [PubMed]
- Park CH, Sung M, Lee GD, et al. Risk of primary spontaneous pneumothorax according to chest configuration. Thorac Cardiovasc Surg 2018;66:583-8. [Crossref] [PubMed]
- Gaunt A, Martin-Ucar AE, Beggs L, et al. Residual apical space following surgery for pneumothorax increases the risk of recurrence. Eur J Cardiothorac Surg 2008;34:169-73. [Crossref] [PubMed]


