The role of rotors in atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice, and case volume is expected to increase as the population ages (1). AF management consists of anticoagulation, rate control, and—for symptomatic patients—antiarrhythmic drugs and ablation to restore sinus rhythm (2). However, the efficacy of antiarrhythmic drugs remains poor, and recent trials challenge the use of pharmacologic therapy to maintain sinus rhythm (3,4). Ablation, too, remains suboptimal, given modest safety (5) and efficacy results (6), even in highly experienced centers.
Since the studies of pulmonary vein ectopy ablation in the treatment of AF (7), the pulmonary veins have been the focus of AF ablation therapy (8-11). Although effective, ablation response rate remains around 50% to 70% single to multi-procedure success rate at 1 year in paroxysmal AF patients (9,10,12) with lower success in persistent AF patients (8). This has prompted a search (8,13) for additional targets of ablation therapy including complex fractionated atrial electrograms (CFAE) (14), whose mechanistic significance remains unclear, or areas of high dominant frequency (15-17) and ganglionated plexus sites (18).
Recently, a growing body of work shows that rotors, identified using near-real-time mapping of AF (19), are both spatially and temporally conserved (20) and thus amenable for ablation (21). Mechanistic proof of concept is supported by the ability of brief targeted rotor ablation alone (22) to eliminate AF acutely (23) and on long-term follow-up in the precise trial. Clinically, Focal Impulse and Rotor Modulation (FIRM) has now been shown by many laboratories to substantially improve the results of AF ablation on long term follow-up in patients with paroxysmal and persistent AF (24,25). The purpose of this review is to discuss the conceptual basis for rotors, evidence for their existence, strategies and pitfalls of rotor mapping, and the role of rotors in guiding substrate-based ablation for AF.
Theory and evidence of rotors
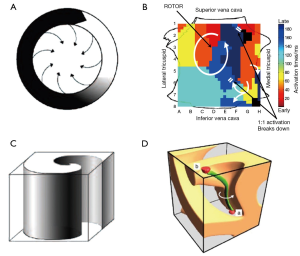
The idea of functional reentry as the driver for AF was first proposed by Lewis in the 1920s (26). Allessie and colleagues subsequently proposed the leading circle theory of reentry (Figure 1A) in the 1970s (27). Rotors altered this concept by proposing a region of extreme wave curvature as the center of reentry where conduction velocity approximates zero and detectable by phase mapping. The first experimental evidence of rotors was reported by Davidenko et al. in 1990 (28), subsequently supported by evidence from modeling and optical mapping of isolated animal heart preparations by Jalife and others (29-31).
With high temporal and spatial resolution, optical mapping and high-density epicardial electrode arrays (32) have been the principal methods by which rotors were explored. Subsequent work using these techniques in a canine model of AF found ablation of rotor sites suppresses subsequent AF inducibility (33). Unfortunately, these methods are impractical for clinical use, and evidence for rotors in human AF was scarce until recently.
Characterizing rotors: from the bench to the bedside
The term “rotor” is applied to a number of different concepts. One accepted definition from the basic literature is a phase singularity whose reverberations radiate “spiral waves” at high speed into surrounding tissue (34). Clinically, the most tangible feature of a rotor is repetitive, cyclic activation around a core (29,30), and while this is the simplest visual criterion for identifying rotors in phase maps (FIRM) or isochronal images, it does not capture the essence of detecting or defining a rotor.
Clinically, while a rotor is superficially similar to reentry around a region of scar, the two mechanisms are quite different. In a reentry around a scar, the central obstacle is ‘inert’ and the surrounding reentrant circuit is the principal mechanism to which ablation is applied. Conversely, for a rotor, the singularity (or ‘core’) is the principal mechanism, while the surrounding ‘spiral waves’ disorganize and fuse passively with the milieu (fibrillatory conduction). Accordingly, therapy is directed to the core. This central difference summarizes why rotors are difficult to detect using approaches that do not take into consideration the impact of repolarization dynamics on wave break from a spiral wave and/or fusion of wavelets from the milieu with spiral waves.
Mechanistically, reentry in a rotor is functional (35) rather than anatomic, and has no [or a highly limited (36)] excitable gap. Because rotor cores exhibit functional reentry, rotors can precess (move) within a defined area as shown in animal models (37) and in 2-3 cm2 areas humans (20). Such precession may contribute to the apparent global disorganization seen in AF.
Another property of rotors is that they are the source of fibrillatory wavefronts (29), and thus “control” surrounding tissue that activates passively, and often via fibrillatory conduction (Figure 1B, arrows to 1:1 breakdown). Such wavefronts can collide/fuse, and exhibit rotational activation within a variable and often small spatial domain. If multiple rotors are present simultaneously, distal wavefronts collide at varying locations, also contributing to the appearance of global disorganization.
Endocardial or epicardial rotors can be projected onto 2-dimensional (2D) movies, or isochronal maps for printed text. Such 2-D projections of rotors are termed spiral waves (38), and are characterized by a small, unexcited core termed a phase singularity. Phase singularities are sites about which all phases of the depolarization/repolarization cycle exist simultaneously, and are important because they identify tissue capable of supporting rotors. First demonstrated by computational modeling (39), 3D rotors are termed scroll waves (Figure 1C), and have recently been shown experimentally (40). In scroll waves, the phase singularity is a linear structure termed a filament (39), about which functional reentry occurs.
Filaments are typically discussed as spanning endocardium to epicardium (I type filaments, Figure 1D) (39), producing spiral waves observable on both surfaces. However, additional configurations are possible including U type filaments with both ends of the filament located on the same surface, and O type filaments in which the filament assumes a closed configuration completely within myocardial tissue (34). For U type and O type filaments, mapping of a surface without a filament terminus shows focal activity (39). Surfaces with two filament ends display contra-rotating spirals and figure-of-8 reentry.
Ionic and structural basis for rotors
A number of ionic changes have been shown to promote the development of rotors in experimental models. Atrial tissue from AF patients demonstrates an up-regulation in IK1 expression (41). Experimentally, transgenic mice overexpressing IK1 demonstrated rapid, stable rotors which were not present in control mice (42). Studies in transfected monolayers of cardiac cells have shown that IKS plays an important role as well (43), promoting rotor formation. Other work has shown that the mild hyperpolarization from enhanced repolarization modifies INa availability (44), altering wavefront conduction. Abnormal calcium dynamics have been implicated in the initiation of torsades des pointes (45), but the precise relationship between ICa and AF rotors remains controversial (46).
Structurally, delayed gadolinium enhancement magnetic resonance imaging (DE-MRI) studies show that scar burden predicts AF recurrence post ablation (47,48), suggesting that AF in patients with atrial scar reflects mechanisms outside the pulmonary veins. Indeed, areas of patchy fibrosis are sites of slow conduction and altered repolarization dynamics that may form and stabilize rotors; in explanted hearts, rotors are predominantly associated with areas of scar and microfibrosis (49) identified histologically. Ongoing clinical studies are examining the relationship between AF rotors identified by FIRM and other techniques and areas of fibrosis on DE-MRI (50).
AF rotor mapping: techniques
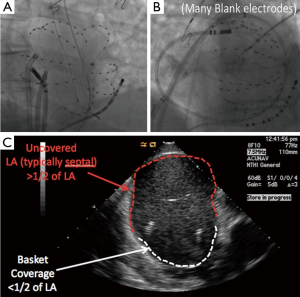
The conventional ablation with or without Focal Impulse and Rotor Modulation (CONFIRM) trial, presented in 2011 (24), demonstrated that rotors and focal sources were present in nearly all patients with paroxysmal, persistent, and long-standing persistent AF, and that ablation of these sources nearly doubled the single-procedure ablation freedom from AF at 1 year (51) and now shown to be durable at 3 years (52). Rotors were identified in near real-time using multielectrode contact basket catheters to record AF (Figure 2) then phase-based algorithms incorporating the dynamic response of repolarization (53-55) and conduction (54,56,57) in these patients to abrupt and gradual changes in rate.
This experimental approach was chosen for a number of reasons. First, although it is theoretically possible to map stationary rotational cycles (e.g., in macro-reentry) with a minimal number of (approximately 4) electrodes (58), fibrillatory rotors precess (i.e., wobble) over time in animal models (37) and humans (20). Figure 3A,B shows the location of the AF rotor core as it precesses in a complex path within a stable region bounded by limited numbers of electrodes. Thus it is necessary to map panoramically (20) to encompass rotor trajectories as completely as possible (Figure 3C).
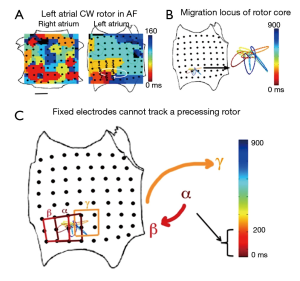
Second, a major obstacle to AF mapping has been separating near field activation from far-field noise in atrial signals. This is particularly important for noncontact mapping, in which electrogram reproducibility decreases with distance from the mapping catheter (59). Earlier work using monophasic action potential catheters demonstrated the importance of accurately determining local activation (60), and thus, biatrial contact mapping was chosen to improve the probability of good quality signals encompassing a significant proportion of the atrial surface (61).
Following signal recording, electrograms are exported to a commercially available computational system (RhythmView, Topera Medical, Palo Alto, CA, USA), which uses phase-based algorithms in conjunction with computational algorithms incorporating repolarization dynamics (54,57,62), conduction dynamics (54,56), and compensation for AF rate oscillations (53) to determine wavefront propagation. Diagnostic movies are then created for physician interpretation and ablation planning. Physician training for movie interpretation is moderate, and includes practice case review and interpretation such that good clinical results can be achieved with a rapid learning curve (25).
Clinical approach and results of FIRM mapping of AF
FIRM mapping identified an average of 2.1±1.0 concurrent rotors or focal sources in the CONFIRM trial (24), that has increased slightly to ≈2.5 sources per patient in recent studies with better basket placement and repeated FIRM maps (25) that were not possible in CONFIRM. In a population of whom two thirds had persistent AF, approximately one-third of sources lie in the right atrium away from the superior vena cava, and hence in regions that would normally not be targeted for ablation. AF rotors and focal sources identified by FIRM are spatiotemporally stable for periods of hours or even months, as described in our early work (61) and in recent external reports from Miller et al. (63). Stability provides a rationale for limited ablation that is less clear for targets that migrate throughout the atria.
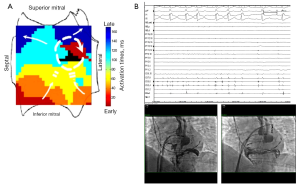
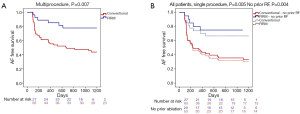
FIRM-guided therapy targets each rotor and focal source for ablation, with the endpoint of rotor/source elimination on repeated FIRM mapping. In the CONFIRM trial (24) and independent external laboratories, elimination of FIRM-sources takes 5-10 minutes per source, for an average of 15-20 minutes FIRM-guided ablation time per case (25). FIRM-guided ablation may terminate AF, typically to sinus rhythm (Figure 4A,B) as first shown outside San Diego by Shivkumar et al. (64) and in a larger series by Kowal et al. (65). However, results of the on-treatment analysis of CONFIRM (21) and the PRECISE trial (22) show that elimination of AF rotors/sources on repeat FIRM mapping is a more effective endpoint. Figure 5A,B shows the 3Y very long term outcome in the CONFIRM trial, in which FIRM + PVI ablation provided substantially higher freedom from AF than conventional ablation after multiple and a single procedure.
AF rotor mapping: pitfalls
A clear technical limitation to AF mapping is electrogram contact as we have reported (19), particularly in atria whose dimensions exceed the size of commercially available basket catheters (Figure 2B) as included in external reports by Shivkumar et al. (64) and Miller et al. (25). While not recommended, successful ablation in such patients is possible when rotor locations coincide with areas of good electrode-tissue apposition—although electrodes opposite these sites will typically then show marginal or poor contact. Multiple basket manipulations can then theoretically be used to sequentially sample regions of the atrium. A second related limitation is poor electrode coverage in the septal aspect of the left atrium (Figure 2C). Future improvements in basket design and maneuverability are required to fully exploit current rotor mapping technology. A final consideration in FIRM ablation is the learning curve for reading the potentially complex maps, although this was relatively rapid in the recent series by Miller et al. (25). Automated detection algorithms are being developed that may further help this process.
Relationship of rotors to CFAE and ganglionated plexi (GP)
There has been a significant amount of interest in CFAE as markers of AF drivers (14). CFAE have multiple definitions, include electrograms with multiple deflections, very short cycle length (<120 ms) (14), or continuous activation (66). Although results using this technique are mixed (67,68), it is frequently considered in patients with persistent or ablation-refractory AF (8). However, the mechanisms of fractionation may be diverse, and a recent study showed poor correlation of AF sources to CFAE (20). This is in agreement with earlier work (69), showing that rotors did not co-localize with regions of fractionation.
Also of interest as potential AF-sustaining sites, GP are regions of autonomic innervation to the atria (18). Prior work has shown that such areas may serve as high frequency AF sources as a result of autonomic remodeling (70). Procedurally, they may be localized by high frequency stimulation and the appearance of a stimulated vagal response, defined as either atrioventricular block, asystole, or an increase in the mean RR interval of greater than or equal to 50% (71). Notably, a randomized clinical trial found that the addition of GP ablation to PVI improved procedural success (72). However, the link between GP and rotors is presently unclear.
Conclusions
Rotors are regions of functional reentry which drive AF. Ionic remodeling, fibrosis, and structural features have been shown to facilitate and stabilize rotor formation, which precess in the midst of complex fibrillatory dynamics making their detection difficult. Confirmation of the existence and importance of rotors in human AF has emerged only recently, with the advent of appropriate procedural and computational techniques. A rapidly growing body of literature shows that rotors are spatially conserved and amenable to ablation. Clinically, studies from multiple independent laboratories shows that rotor elimination via FIRM substantially improves AF freedom compared to conventional ablation alone. Future clinical studies should confirm these promising results in multicenter randomized trials, which are underway. Mechanistically, studies should define how rotors anchor in human atria, and the mechanisms for fibrillatory conduction.
Acknowledgements
This work was supported by grants to DEK from the American Heart Association (BGIA) and NIH (HL83359) and to SMN from the NIH (HL70529, HL83359, and HL103800) and the Doris Duke Charitable Foundation. The authors would like to acknowledge Donna Cooper, RN, Elizabeth Greer, RN, Stephanie Yoakum, RN, Judy Hildreth, RN, Cherie Jaynes, RN, Kenneth Hopper, CVT, and Tony Moyeda, CVT for their contribution to this work.
Disclosure: Dr. Krummen has received grant support from the American Heart Association (BGIA) and the NIH (HL83359). He reports consulting fees from Insilicomed and Topera Inc. His institution has received fellowship support from Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude Medical. Dr. Swarup has received consulting fees/honoraria from Biosense Webster and research grants from Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude Medical. Dr. Narayan has received support from the NIH (HL83359, HL103800). Dr. Narayan is co-author of intellectual property owned by the University of California Regents and licensed to Topera Inc. Dr. Narayan has received consulting fees from the American College of Cardiology Foundation, Abbott Inc. and Medtronic Inc., and royalty income from UpToDate.
References
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5. [PubMed]
- Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. [PubMed]
- Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667-77. [PubMed]
- Saksena S, Slee A, Waldo AL, et al. Cardiovascular outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management). An assessment of individual antiarrhythmic drug therapies compared with rate control with propensity score-matched analyses. J Am Coll Cardiol 2011;58:1975-85. [PubMed]
- Bohnen M, Stevenson WG, Tedrow UB, et al. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm 2011;8:1661-6. [PubMed]
- Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol 2011;57:160-6. [PubMed]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [PubMed]
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012;9:632-696.e21.
- Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333-40. [PubMed]
- Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713-23. [PubMed]
- Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med 2006;354:934-41. [PubMed]
- Morillo CA, Verma A, Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311:692-700. [PubMed]
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002;415:219-26. [PubMed]
- Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044-53. [PubMed]
- Habel N, Znojkiewicz P, Thompson N, et al. The temporal variability of dominant frequency and complex fractionated atrial electrograms constrains the validity of sequential mapping in human atrial fibrillation. Heart Rhythm 2010;7:586-93. [PubMed]
- Krummen DE, Peng KA, Bullinga JR, et al. Centrifugal gradients of rate and organization in human atrial fibrillation. Pacing Clin Electrophysiol 2009;32:1366-78. [PubMed]
- Atienza F, Almendral J, Jalife J, et al. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 2009;6:33-40. [PubMed]
- Scherlag BJ, Nakagawa H, Jackman WM, et al. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol 2005;13:37-42. [PubMed]
- Narayan SM, Krummen DE, Rappel WJ. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:447-54. [PubMed]
- Narayan SM, Shivkumar K, Krummen DE, et al. Panoramic electrophysiological mapping but not electrogram morphology identifies stable sources for human atrial fibrillation: stable atrial fibrillation rotors and focal sources relate poorly to fractionated electrograms. Circ Arrhythm Electrophysiol 2013;6:58-67. [PubMed]
- Narayan SM, Krummen DE, Clopton P, et al. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation). J Am Coll Cardiol 2013;62:138-47. [PubMed]
- Narayan SM, Krummen DE, Donsky A, et al. Treatment Of Paroxysmal Atrial Fibrillation By Targeted Elimination Of Stable Rotors And Focal Sources Without Pulmonary Vein Isolation: The Precise Rotor Elimination Without Concomitant Pulmonary Vein Isolation For Subsequent Elimination Of PAF (PRECISE) Trial. Heart Rhythm 2013;10:1414-LB01-4-5 (abstract).
- Narayan SM, Patel J, Mulpuru S, et al. Focal impulse and rotor modulation ablation of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on follow-up: a video case study. Heart Rhythm 2012;9:1436-9. [PubMed]
- Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628-36. [PubMed]
- Miller JM, Kowal RC, Swarup V, et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol 2014;25:921-9. [PubMed]
- Lewis ST. eds. The Mechanism and Graphic Registration of the Heart Beat. 3rd ed. Chicago: Chicago Medical Book Company, 1924.
- Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res 1977;41:9-18. [PubMed]
- Davidenko JM, Kent PF, Chialvo DR, et al. Sustained vortex-like waves in normal isolated ventricular muscle. Proc Natl Acad Sci U S A 1990;87:8785-9. [PubMed]
- Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature 1998;392:75-8. [PubMed]
- Davidenko JM, Pertsov AV, Salomonsz R, et al. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature 1992;355:349-51. [PubMed]
- Gray RA, Jalife J, Panfilov AV, et al. Mechanisms of cardiac fibrillation. Science 1995;270:1222-3. [PubMed]
- Nash MP, Mourad A, Clayton RH, et al. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation 2006;114:536-42. [PubMed]
- Chou CC, Chang PC, Wen MS, et al. Epicardial ablation of rotors suppresses inducibility of acetylcholine-induced atrial fibrillation in left pulmonary vein-left atrium preparations in a beagle heart failure model. J Am Coll Cardiol 2011;58:158-66. [PubMed]
- Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res 2013;112:849-62. [PubMed]
- Vaquero M, Calvo D, Jalife J. Cardiac fibrillation: from ion channels to rotors in the human heart. Heart Rhythm 2008;5:872-9. [PubMed]
- Kirchhof C, Chorro F, Scheffer GJ, et al. Regional entrainment of atrial fibrillation studied by high-resolution mapping in open-chest dogs. Circulation 1993;88:736-49. [PubMed]
- Zlochiver S, Yamazaki M, Kalifa J, et al. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm 2008;5:846-54. [PubMed]
- Jalife J. Déjà vu in the theories of atrial fibrillation dynamics. Cardiovasc Res 2011;89:766-75. [PubMed]
- Berenfeld O, Pertsov AM. Dynamics of intramural scroll waves in three-dimensional continuous myocardium with rotational anisotropy. J Theor Biol 1999;199:383-94. [PubMed]
- Yamazaki M, Mironov S, Taravant C, et al. Heterogeneous atrial wall thickness and stretch promote scroll waves anchoring during atrial fibrillation. Cardiovasc Res 2012;94:48-57. [PubMed]
- Dobrev D, Wettwer E, Himmel HM, et al. G-Protein beta(3)-subunit 825T allele is associated with enhanced human atrial inward rectifier potassium currents. Circulation 2000;102:692-7. [PubMed]
- Li J, McLerie M, Lopatin AN. Transgenic upregulation of IK1 in the mouse heart leads to multiple abnormalities of cardiac excitability. Am J Physiol Heart Circ Physiol 2004;287:H2790-802. [PubMed]
- Muñoz V, Grzeda KR, Desplantez T, et al. Adenoviral expression of IKs contributes to wavebreak and fibrillatory conduction in neonatal rat ventricular cardiomyocyte monolayers. Circ Res 2007;101:475-83. [PubMed]
- Hou L, Deo M, Furspan P, et al. A major role for HERG in determining frequency of reentry in neonatal rat ventricular myocyte monolayer. Circ Res 2010;107:1503-11. [PubMed]
- Choi BR, Burton F, Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physiol 2002;543:615-31. [PubMed]
- Warren M, Huizar JF, Shvedko AG, et al. Spatiotemporal relationship between intracellular Ca2+ dynamics and wave fragmentation during ventricular fibrillation in isolated blood-perfused pig hearts. Circ Res 2007;101:e90-101. [PubMed]
- Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758-67. [PubMed]
- Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498-506. [PubMed]
- Nair K, Umapathy K, Farid T, et al. Intramural activation during early human ventricular fibrillation. Circ Arrhythm Electrophysiol 2011;4:692-703. [PubMed]
- McGann CJ, Kholmovski EG, Oakes RS, et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol 2008;52:1263-71. [PubMed]
- Narayan SM, Hassankhani A, Feld GK, et al. Separating non-isthmus- from isthmus-dependent atrial flutter using wavefront variability. J Am Coll Cardiol 2005;45:1269-79. [PubMed]
- Narayan SM, Baykaner T, Clopton P, et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol 2014;63:1761-8. [PubMed]
- Narayan SM, Krummen DE, Kahn AM, et al. Evaluating fluctuations in human atrial fibrillatory cycle length using monophasic action potentials. Pacing Clin Electrophysiol 2006;29:1209-18. [PubMed]
- Narayan SM, Kazi D, Krummen DE, et al. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: a mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol 2008;52:1222-30. [PubMed]
- Narayan SM, Franz MR, Clopton P, et al. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation 2011;123:2922-30. [PubMed]
- Lalani GG, Schricker A, Gibson M, et al. Atrial conduction slows immediately before the onset of human atrial fibrillation: a bi-atrial contact mapping study of transitions to atrial fibrillation. J Am Coll Cardiol 2012;59:595-606. [PubMed]
- Krummen DE, Bayer JD, Ho J, et al. Mechanisms of human atrial fibrillation initiation: clinical and computational studies of repolarization restitution and activation latency. Circ Arrhythm Electrophysiol 2012;5:1149-59. [PubMed]
- Rappel WJ, Narayan SM. Theoretical considerations for mapping activation in human cardiac fibrillation. Chaos 2013;23:023113. [PubMed]
- Thiagalingam A, Wallace EM, Boyd AC, et al. Noncontact mapping of the left ventricle: insights from validation with transmural contact mapping. Pacing Clin Electrophysiol 2004;27:570-8. [PubMed]
- Narayan SM, Wright M, Derval N, et al. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm 2011;8:244-53. [PubMed]
- Narayan SM, Krummen DE, Enyeart MW, et al. Computational mapping identifies localized mechanisms for ablation of atrial fibrillation. PLoS One 2012;7:e46034. [PubMed]
- Narayan SM, Lindsay BD, Smith JM. Demonstration of the proarrhythmic preconditioning of single premature extrastimuli by use of the magnitude, phase, and distribution of repolarization alternans. Circulation 1999;100:1887-93. [PubMed]
- Miller JM, Kowal RC, Swarup V, et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol 2014;25:921-9. [PubMed]
- Shivkumar K, Ellenbogen KA, Hummel JD, et al. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol 2012;23:1277-85. [PubMed]
- Kowal RC, Daubert J, Day J, et al. Results of focal impulse and rotor modulation (FIRM) for atrial fibrillation are equivalent between patients treated in San Diego compared with sites new to FIRM ablation: an extended multi-center experience. Heart Rhythm 2013;10:S479 abstract.
- Haïssaguerre M, Sanders P, Hocini M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol 2005;16:1125-37. [PubMed]
- Li WJ, Bai YY, Zhang HY, et al. Additional ablation of complex fractionated atrial electrograms after pulmonary vein isolation in patients with atrial fibrillation: a meta-analysis. Circ Arrhythm Electrophysiol 2011;4:143-8. [PubMed]
- Oral H, Chugh A, Yoshida K, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol 2009;53:782-9. [PubMed]
- Mandapati R, Skanes A, Chen J, et al. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation 2000;101:194-9. [PubMed]
- Po SS, Scherlag BJ, Yamanashi WS, et al. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm 2006;3:201-8. [PubMed]
- Verma A, Saliba WI, Lakkireddy D, et al. Vagal responses induced by endocardial left atrial autonomic ganglion stimulation before and after pulmonary vein antrum isolation for atrial fibrillation. Heart Rhythm 2007;4:1177-82. [PubMed]
- Katritsis DG, Giazitzoglou E, Zografos T, et al. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm 2011;8:672-8. [PubMed]


