Repair of post-intubation tracheoesophageal fistulae through the left pre-sternocleidomastoid approach: a recent case series of 13 patients
Introduction
Post-intubation tracheoesophageal fistula (TEF) is a rare complication of tracheotomy occurring in less than 1% of patients undergoing tracheotomy (1,2). The formation of TEF is mainly attributed to the erosion of the posterior tracheal wall from excessive cuff pressure or damage of the posterior tracheal wall during percutaneous dilational tracheotomy (1-3). A right angle of the tracheal tube itself can also cause undue pressure against the posterior tracheal wall that could lead to erosion (3). The existence of a nasogastric tube within the esophagus and tracheal inflammation are considered to be additional factors which further contribute to the TEF formation (1-3).
Surgical intervention is the only viable option for the repair of TEF, as spontaneous healing is not expected even in very small fistulae (4). Stenting of the esophagus fails to permanently heal the fistula and it could be used only as a temporary solution before surgical repair (5). The optimal surgical access and surgical intervention for the repair of TEF depends on various factors, such as local conditions, extent of fistula, presence of circumferential tracheal stenosis or tracheomalacia and dependence of the patient from mechanical ventilation (6,7). Left pre-sternocleidomastoid approach is a simple approach for the repair of post-intubation TEF in cases which are not complicated with tracheal stenosis. Pre-sternocleidomastoid approach permits easy access to perform direct closure of the fistula which should always followed by interposition of the whole pedicled left sternocleidomastoid muscle (SCMM) between the two suture lines to avoid fistula recurrence. A recent case series of 13 post-intubation TEFs that underwent direct closure and SCMM interposition with the pre-sternocleidomastoid approach is reported in order to discuss the advantages and drawbacks of this approach for the surgical repair of post-intubation TEFs.
Patient and methods
Patients
Thirteen patients with a mean age of 54.1±12.6 years (eight males) were admitted for the repair of post-intubation TEF during the recent 7 years period, from January 2007 to December 2013. All the 13 TEFs were the result of prolonged mechanical ventilation through a cuffed tracheotomy tube in critically ill patients. Two more patients who underwent post-laryngectomy TEF repair during the study period were not included in the study. The reason to proceed with prolonged mechanical ventilation and tracheotomy in the 13 patients was complicated coronary artery bypass grafting (GABG) procedures in 4 patients, pulmonary infection in 3 patients with immunosupression or exacerbation of chronic heart failure and chronic obstructive pulmonary respectively, multiple trauma in 2, cerebrovascular accident in 2, ventricular tachycardia/fibrillation in 1 patient with chronic heart failure and chocking in the last 1.
Tracheotomies were performed in an elective basis while 12 out of the 13 tracheotomies were performed with the percutaneous dilational technique. Technical problems during the creation of a percutaneous dilational tracheotomy were reported in 3 patients who developed early post-tracheotomy TEF that was definitely diagnosed on 6th, 10th and 11th post-tracheotomy day respectively.
A self-expandable, covered esophageal stent was initially inserted to eliminate aspiration of gastric content and saliva through the fistula in two out of the 13 patients. The stent was removed immediately since the final decision to proceed with surgical repair.
Diagnosis and preparation for surgery
Diagnosis of TEF was made in all the 13 patients while on mechanical ventilation. The prominent clinical symptom which made the suspicion of TEF was the evidence of aspiration of gastric content within the tracheobronchial tree or the evidence of air leak around the cough of the tracheal tube with abdominal distention. Confirmation of the clinical diagnosis was made through esophagoscopy where the cuff of the tracheal tube was found to protrude within the esophageal lumen through the fistulous tract. Fiberoptic bronchoscopy through both routes, the vocal cords and the tracheotomy opening, was always made to define the exact site and length of the fistulae and to exclude concomitant tracheal or laryngeal stenosis, extensive tracheomalacia or tracheal wall inflammation. Since the detection of TEF an endoscopic gastroscopy was made to diminish reflux and soiling of the bronchial tree with gastric contents and to improve or to maintain the nutritional status of the patient together with the administration of parenteral nutrition. None of the 13 patients underwent feeding jejunostomy. The cuff of an adjustable tracheotomy tube was advanced under bronchoscopic guidance below the fistulous tract with its tip reaching just above the carina in order to avoid aspiration of gastric content and to secure also effective mechanical ventilation. All patients received wide spectrum antibiotics to treat or to prevent pulmonary infections.
Surgical procedure and postoperative care
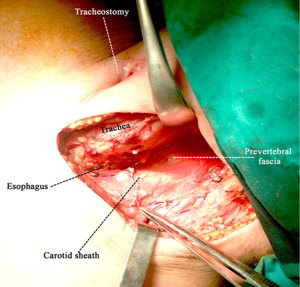
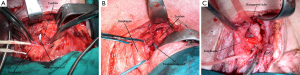
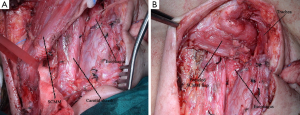
The patient lied supine in the operative bed with its neck hyperextended and turned to the non-operating (right) side. A left pre-sternocleidomastoid incision starting 2-3 cm below the mastoid process and extending down to the sternal notch was made. The prevertebral fascia was reached by dissecting the space between the larynx, trachea and thyroid gland in one side and the carotid sheath and SCMM in the other. Ligation of the middle thyroid vein, inferior thyroid artery and omohyoideus muscle was required to gain access to the prevertebral fascia (Figure 1). The fistulae were detected almost always 1-3 cm below the tracheotomy opening and were divided, separating this way the esophagus from membranous trachea. Dissection of the fistulous tract was made closer to the esophagus in order to leave a fibrous rim of the fistulous tract attached to the membranous trachea opening. At that time the esophagus was blindly dissected down to the mediastinum, mobilized and encircled with tapes (Figure 2). Closure of the esophagus was made in two layers with absorbable polyglactin 4.0 sutures and closure of the membranous trachea with non-absorbable monofilament 4.0 sutures. The whole SCMM was then mobilized by cutting-off its insertion to the mastoid process. The fully mobilized SCMM was then positioned between the suture line of trachea and esophagus (Figure 3) and fixed to the prevertebral fascia with two or three polyglactin 2.0 sutures (Figure 4).
Results
The elapsed time from the creation of tracheotomy to the detection of TEF varied from 6 to 59 days (mean: 33.7±19.8 days, median: 35 days). Fiberoptic bronchoscopy did not reveal any concomitant tracheal or laryngotracheal stenosis in those 13 patients. Five out of the 13 procedures were performed in mechanically ventilated critically ill patients, because TEF-associated respiratory complications prevented effective weaning from mechanical ventilation or even effective mechanical ventilation due to the escape of large amount of air through the fistulous tract. In one out of the above mentioned 5 cases recurrent episodes of air flow obstruction were noted because of tracheal tube displacement within the fistula during clearance of bronchial secretions, requiring repeated bronchoscopies for tube repositioning above the carina.
The overall mortality rate was 23.07% in the current series (3 out of the 13 patients). Fatal outcome occurred in patients who underwent fistula repair while on mechanical ventilation for serious complications of CABG therefore the mortality rate after TEF repair in mechanically ventilated patients was 60%. Fatal outcome in those three patients was the result of sepsis and multiple organ failure related to the general condition of the patient and not to surgery for TEF repair. Recurrence of the fistula was observed in one out of those three patients. Failure of the repair in this particular case was attributed to the extent of the fistula (estimated length >3 cm), to the extensive inflammation of the whole tracheal wall and to the small volume of the left SCMM because of myatrophy. SCMM was found at surgery to be seriously affected by the ICU myopathy.
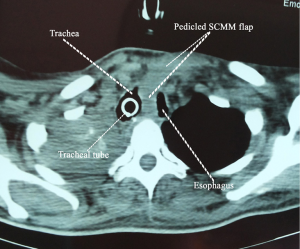
The results of TEF repair were favorable in the rest eight patients who underwent fistula repair after their final weaning from mechanical ventilation. One out of those eight patients required the additional procedure of temporary T-tube insertion in the 3rd post-repair day because of the existence of extensive tracheomalacia which affected breathing. The T-tube was removed six months later and the patient did not experience any other breathing problems. Permanent left vocal cord paralysis was noted in one out of the 10 survivors (10%). Temporary swallowing problems were also noted in two patients (20%) that completely subsided within 2 months after TEF repair.
Control bronchoscopies were performed for various reasons in 6 out of the 10 survivors within 3 to 12 months after TEF repair. A normal tracheal lumen was found with limited scarring in the upper posterior tracheal wall.
Discussion
Benign TEFs are the result of many different etiologies. Prolonged mechanical ventilation with a cuffed tube, previous tracheal or esophageal surgery, granulomatous mediastinal infections, use of indwelling tracheal or esophageal stents, trauma, iatrogenic injuries, and caustic ingestion are the commonest etiologies of TEF formation (6-11). The majority of these fistulae are the result of prolonged mechanical ventilation, indeed in recent reports the etiology seems to be more diverse, including more and more complex cases after esophagectomy and laryngectomy (6,8).
TEF develops usually after 12-200 days of mechanical ventilation with a mean of 42 days (4). Similar findings were observed in the current series where the median time from the creation of tracheotomy to TEF detection was 35 days. We have to note here that in the current series three early post-tracheotomy TEFs are reported as the result of posterior tracheal wall laceration during percutaneous tracheotomy. Early development of post-tracheotomy TEF is mainly related to the damage to the posterior membranous tracheal wall and possibly to the esophagus during the creation of tracheotomy, especially with the percutaneous dilational technique (2,12). Bronchoscopic guidance is recommended to be routinely performed during tracheotomy to avoid puncture of the posterior tracheal wall (2,13), however, some experienced teams do not advocate its routine use (14).
The technique of choice for the repair of the rare post-intubation TEFs still remains controversial (6,7). The post-intubation fistula is usually accessed through a cervical incision, while a median upper or full sternotomy is mandated in few cases. Direct, one-stage suture closure of the fistula, segmental tracheal resection with primary anastomosis and direct esophageal closure, closure of the defects with soft tissue flaps, a 2-stage approach with esophageal diversion and primary closure of the tracheal defect followed by esophageal replacement and finally, stenting of the esophagus with covered self-expandable metallic stents have been used in the past for TEF repair. The choice depends on the local conditions, the dependence of the patient from mechanical ventilation and the general condition of the patient (4,6-11,15). Studies concerning surgical repair are limited to the experience of very few references group, therefore the existing evidence is at the moment that of the expert’s opinion (8-10). According to the opinion of these leading groups from Boston and Paris, the best way to manage a TEF is by the Grillo’s technique which is shortly described as tracheal resection including the fistulous tract and repair of the esophagus in two layers irrespective to the presence of tracheal stenosis or not (8-10). Circumferential tracheal stenosis was reported to be implicated in 28-75% of post-intubation TEF cases in previously published case-series (9-11,15). None of the 13 patients in the current series had a concomitant tracheal or laryngotracheal stenosis, with the exception of one patient who had tracheomalacia and underwent temporary stenting of his trachea.
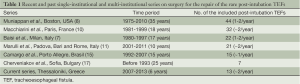
Direct closure of a post-intubation TEF through a pre-sternocleidomastoid approach was employed in 28% to 83.8% of cases in different case-series (7,8,10,11). The pre-sternocleidomastoid approach offers very good access for the repair of post-intubation TEF which usually resides in the lower neck or very high in the mediastinum (7,16,17). According to our experience obtained from the recent case-series of 13 consecutive cases—which is one of the largest single-institutional series on the repair of post-intubation TEFs in the contemporary literature (Table 1)—the important points during direct repair and closure of post-intubation TEF are: first, the full mobilization of the esophagus since the dissection of the fistulous tract and the extensive separation of esophagus from trachea; and second, the interposition of the whole pedicled SCMM between the two suture lines; the muscle should be fixed to the prevertebral fascia. The repair is very durable and tough and the patient can be mechanically ventilated after fistula repair until final weaning. In their recent report, Shen et al. [2010] do not consider ventilator-dependence to be a contraindication for TEF repair, particularly if tracheal resection and reconstruction are not required (6). We have to mention however that TEF repair is best accomplished in non-ventilator dependent patient. Interposition of the muscle flap is of great importance to avoid recurrence of the fistula and to permit mechanical ventilation through an adjustable cuffed tracheal tube which should be advanced low in the trachea under bronchoscopic guidance (9-11,18). In addition, the left pre-sternocleidomastoid approach can easily be converted to a U shaped incision if required from the local conditions to perform tracheal resection (16). We have to note here that the length of the fistula cannot be accurately estimated before the repair with either, bronchoscopy or esophagoscopy. The length of the fistula is usually overestimated during esophagoscopy because of the dilatation of the tract by the protruding inflated cuff of the tracheal tube and underestimated during bronchoscopy. The proposed by the reference-groups tracheobronchial resection should include the tracheotomy site and the site of the fistula, thus a long tracheal segment has to be resected in most cases. In contrary to the series published by Muniappan, Macchiarini and Camargo (8,10,15), tracheal resection was seldom necessary in the 22 patients with post-intubation TEF in the series published by Baisi et al. [1999], results that are in agreement with the results of the current study (7).
Full table
Mortality rates from 0% to 10.5% have been reported in the past after TEF repair, however, mortality rates are not comparable because of the heterogeneity of the included cases in each of the case-series. Mortality was related to septic complications due mainly to fistula recurrence or to the poor overall condition of the patient (4,6-11,17). Mortality of TEF repair through the left pre-sternocleidomastoid approach in the current series was 23.07%. All deaths were observed in patients who underwent TEF repair while on mechanical ventilation and had pulmonary sepsis. We can speculate therefore that mortality was not related to the procedure for TEF repair itself, with the exception of one case with fistula recurrence.
The single-stage repair of post-intubation TEFs with the pre-sternocleidomastoid approach carries the risk of injuring the left recurrent laryngeal nerve. Injury to the nerve when direct closure of the fistula is attempted through the left pre-sternocleidomastoid approach varies from 4-16% (10% in the current series), indeed injury to the recurrent nerve is possible with any other technique of TEF repair (7,9-11). The injury to the nerve is usually related to damage from electrocautery or from the pressure applied by the retractors (Figure 1) (7).
In conclusion, the direct one-stage closure of trachea and esophagus with SCMM interposition through the left pre-sternocleidomastoid approach is very effective and easily reproducible technique for the repair of post-intubation TEFs, even in mechanically ventilated patients. Mobilization of the esophagus to a large extent after dissection of the fistulous tract and interposition of the whole SCMM between the suture lines makes a very durable construction which is able to prevent fistula recurrence and to be applied in mechanically ventilated patients. The main drawbacks of the technique are the risk to damage the left recurrent laryngeal nerve and the unsuitability of the technique when TEF is further complicated by a concomitant tracheal or laryngotracheal stenosis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Heffner JE, Miller KS, Sahn SA. Tracheostomy in the intensive care unit. Part 2: Complications. Chest 1986;90:430-6. [PubMed]
- Epstein SK. Late complications of tracheostomy. Respir Care 2005;50:542-9. [PubMed]
- De Leyn P, Bedert L, Delcroix M, et al. Tracheotomy: clinical review and guidelines. Eur J Cardiothorac Surg 2007;32:412-21. [PubMed]
- Marzelle J, Dartevelle P, Khalife J, et al. Surgical management of acquired post-intubation tracheo-oesophageal fistulas: 27 patients. Eur J Cardiothorac Surg 1989;3:499-502; discussion 502-3. [PubMed]
- Eleftheriadis E, Kotzampassi K. Temporary stenting of acquired benign tracheoesophageal fistulas in critically ill ventilated patients. Surg Endosc 2005;19:811-5. [PubMed]
- Shen KR, Allen MS, Cassivi SD, et al. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg 2010;90:914-8; discussion 919. [PubMed]
- Baisi A, Bonavina L, Narne S, et al. Benign tracheoesophageal fistula: results of surgical therapy. Dis Esophagus 1999;12:209-11. [PubMed]
- Muniappan A, Wain JC, Wright CD, et al. Surgical treatment of nonmalignant tracheoesophageal fistula: a thirty-five year experience. Ann Thorac Surg 2013;95:1141-6. [PubMed]
- Mathisen DJ, Grillo HC, Wain JC, et al. Management of acquired nonmalignant tracheoesophageal fistula. Ann Thorac Surg 1991;52:759-65. [PubMed]
- Macchiarini P, Verhoye JP, Chapelier A, et al. Evaluation and outcome of different surgical techniques for postintubation tracheoesophageal fistulas. J Thorac Cardiovasc Surg 2000;119:268-76. [PubMed]
- Marulli G, Loizzi M, Cardillo G, et al. Early and late outcome after surgical treatment of acquired non-malignant tracheo-oesophageal fistulae. Eur J Cardiothorac Surg 2013;43:e155-61. [PubMed]
- Glossop A, Meekings TC, Hutchinson SP, et al. Complications following Tracheostomy Insertion in Critically Ill Patients -Experience from a Large Teaching Hospital. Journal of the Intensive Care Society 2011;12:301-6.
- Kluge S, Baumann HJ, Maier C, et al. Tracheostomy in the intensive care unit: a nationwide survey. Anesth Analg 2008;107:1639-43. [PubMed]
- Jackson LS, Davis JW, Kaups KL, et al. Percutaneous tracheostomy: to bronch or not to bronch--that is the question. J Trauma 2011;71:1553-6. [PubMed]
- Camargo JJ, Machuca TN, Camargo SM, et al. Surgical treatment of benign tracheo-oesophageal fistulas with tracheal resection and oesophageal primary closure: is the muscle flap really necessary? Eur J Cardiothorac Surg 2010;37:576-80. [PubMed]
- Santini P, Dragotto A, Gigli PM, et al. Postintubation tracheoesophageal fistula: surgical treatment of three cases. J Thorac Cardiovasc Surg 1998;116:518-9. [PubMed]
- Cherveniakov A, Tzekov C, Grigorov GE, et al. Acquired benign esophago-airway fistulas. Eur J Cardiothorac Surg 1996;10:713-6. [PubMed]
- Golash V. Single-stage repair of a large acquired tracheoesophageal fistula with interposition of 2 muscle pedicle flaps and laparoscopic gastrojejunostomy. J Thorac Cardiovasc Surg 2006;131:1413-4. [PubMed]


