
Insights into Friedman stage II and III OSA patients through drug-induced sleep endoscopy
Introduction
Obstructive sleep apnea (OSA) is associated with ailments such as socially unacceptable snoring, choking, frequent awakening, daytime sleepiness, fatigue, and comorbidities, such as hypertension (1), cardiovascular disease (2), cerebrovascular disease (3), diabetes (4), and overall increased mortality (5). Nocturnal intermittent hypoxia (NIH), sleep fragmentation, inflammation, increased sympathetic activity are considered as pathophysiological mechanisms contributing to general injuries (6-8). The prevalence is increasing from 3–7% to 13% in men population and 2–5% to 6% in women (9,10), and becoming a public health care issue.
Continuous positive airway pressure (CPAP) therapy is generally considered as the first-choice treatment for OSA, providing air pressure support to the upper airway (UA) lumen to relieve collapse. As a result, CPAP has been shown to improve sleepiness, increase quality of life (QOL) and decrease comorbidities (11-13). However, 46–83% do not tolerate CPAP or present an inadequate adaptation to it (14), and surgery is a reasonable and necessary alternative therapy. Uvulopalatopharyngoplasty (UPPP) was initially performed by Ikematsu (15) in 1952 for snoring, and introduced for OSA by Fujita (16), and still is the most commonly performed surgical procedure, however, its “success rate” or “response rate” has been described as limited and variable, ranging from 36% (17) to 78% (18) based on greater than 50% apnea hypopnea index (AHI) reduction and post-surgical AHI less than 20 (19). UA pressure measurements and endoscopy findings showed that patients with hypopharyngeal obstruction had a high percentage of relapses (20,21).
The Friedman staging system has been described as a simple and effective method to predict surgical outcomes according to the tongue-palate position relationship, tonsil size, craniofacial anatomy and body mass index (BMI) (22,23). It has been shown that hypopharyngeal obstruction, and therefore worse surgical outcomes for palatal surgery was associated with increase in staging, with 80.6% to stage I to 37.9% in stage II and 8.1% to stage III, respectively (24). A similar result was also described by Li et al. with a 96% UPPP successful rate in stages II vs. 65% in stage III (25). Although stage III subjects are thought to have less UA volume than stage II and higher proportion of retroglossal obstruction by pressure manometry (26), detailed differences about collapse characteristics across stages were not described in previous studies and need to be further explored.
Drug-induced sleep endoscopy (DISE) can be useful to evaluate obstructive airway characteristics during sleep (27) and its repeatability (28) and reliability (29,30) have been extensively described. Moreover, DISE has been more sensitive in finding hypopharyngeal collapse compared to awake endoscopy (31-33). To our knowledge, there are no studies assessing UA obstruction characteristics of different Friedman stages based on DISE. The objective of this study is to compare Friedman’s stages II and III and DISE findings in patients with OSA, therefore helping in fulfill an important knowledge gap on the correlation between awake and sedated UA examination.
Methods
Study design
Retrospective case series of OSA patients who underwent DISE at a tertiary referral center between June 2013 and May 2017. Inclusion criteria were subjects who: (I) had been diagnosed by in-laboratory polysomnography (PSG) or home based sleep test, with apnea-hypopnea index (AHI) ≥5 events/hour; (II) were intolerant to CPAP and who performed DISE to evaluate further therapy; (III) presented Friedman Staging System II or III. Patients who had previous history of UA procedures for sleep breathing disordered, including UPPP, palatal radiofrequency, genial tubercle advancement, tongue base reduction, maxillo-mandibular advancement or orthognathic surgeries, were excluded. Demographic and clinical features including age, gender, body mass index (BMI), Epworth Sleepiness Scale (ESS) score, tonsil size, Friedman tongue position (FTP); sleep study outcomes including AHI, oxygen desaturation index (ODI), lowest oxygen saturation (LSAT); DISE findings; and the surgeries performed after DISE and the polysomnographic variables after surgery performed between 3 to 6 months were extracted from Stanford’s Redcap and EPIC databases. Patients were divided into two groups: stage II and stage III, baseline characteristics, DISE findings and surgeries outcomes were compared, and associations were analyzed in all subjects.
The trial was conducted in accordance with the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the Institutional Review Board and Hospital Research Ethics Committee of Stanford Hospital and Clinics (approval number 35054). All patients enrolled completed the informed consent form.
Sleep studies
Sleep studies were scored in accordance to the American Academy of Sleep Medicine 2012 edition. Apnea was identified when the amplitude of the airflow decreased ≥90% for ≥10 seconds. Hypopnea was identified when airflow amplitude decreased ≥30% for ≥10 seconds and was associated with oxygen desaturation ≥3% or an arousal. ODI represented the number of events of oxygen desaturation ≥3% per hour. LSAT represented the lowest oxygen saturation during sleep.
Friedman staging system
Only individuals in stages II and III were included. Stage II presented tonsil size 0, 1, or 2 with FTP I or II (stage IIa), or tonsil size 3 or 4 with FTP III or IV (stage IIb), and BMI <40. Stage III is tonsil size 0, 1, or 2 with FTP III or IV, and BMI<40 (23).
DISE protocol
The patients were placed in supine position in the operating room. Nasal decongestion oxymetazoline and topical lidocaine gel was applied to nasal valve area prior to endoscopy. Dexmedetomidine was the sedative agent, administered with an IV bolus at 1.5 mcg/kg over 10 minutes, followed by a maintenance infusion rate of 1.5 mcg/kg/h (34). An Olympus® flexible endoscope with a 3.2-mm diameter was inserted into the nose, UA obstructive subsites of velum (V), oropharynx (O), tongue (T), epiglottis (E) and levels of upper: velum and/or oropharynx (V/O); lower: tongue base and/or epiglottis (T/E); combined: V/O + T/E were observed. The assessment began after the first cycle of snoring and obstruction completed. The cycle is here defined as a complete and stable sequence of snoring-obstructed breathing, or desaturations. At least 2 cycles of obstructed breathing were observed for each subsite, as recommended by the European position paper on DISE (35). The duration of evaluation was 15 to 20 minutes. The grade and patterns of UA collapse were described by Velum oropharynx tongue base epiglottis (VOTE) classification (36). All evaluations were performed and classified exclusively by a senior and experienced surgeon (R.C).
Statistical analysis
Continuous variables were summarized with mean ± standard deviation, meanwhile some variables (AHI, ODI, LSAT, ESS) lack of normality distribution were aware and median (range) were demonstrated. Categorical variables were summarized with frequencies and percentages. Continuous variables were compared using Student t-test when normally distributed or Wilcoxon rank sum test to another way. Categorical variables were compared using Fisher’s exact test or Wilcoxon rank-sum test when categories were ordered. Logistical regression model was used to analyze the association between stage II/III and single/multiple obstructive sites; isolated upper or lower level /combined levels obstruction; non (0: <50%), partial (1: 50–75%) and complete (2: <75%) collapse; 0/1+2 collapse in subsites with differential collapse patterns. All models were presented no adjustment and adjustment for age, gender, BMI. All the analyses were performed by SAS 9.4 (SAS Inc, Cary, NC, USA). P<0.05 was considered statistically significant.
Results
The study assessed 193 records. Eighteen patients were excluded as they underwent prior surgical procedures for OSA. Baseline characteristics including age, BMI, ESS, AHI, ODI, LSAT were compared across groups and no significant differences were found as shown in Table 1.

Full table
Collapse pattern and obstruction degree in VOTE showed no significant difference between the two groups; there was no significant association between stage II or III, obstruction sites, single or multiple sites obstruction, isolated or combined levels obstruction, in either unadjusted or adjusted logistic regression models, for partial or complete collapse (Tables 2,3).
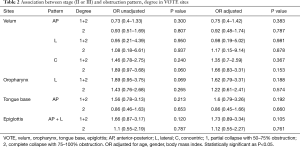
Full table
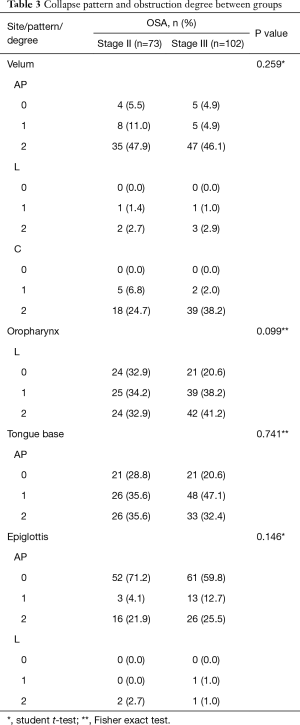
Full table
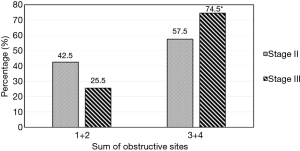
There was a significant higher proportion (74.5%) of more than 2 obstructive sites in stage III patients than in stage II patients (57.5%), P=0.022 (Figure 1). However, there was no significant difference in the distribution of obstruction levels between the stages as shown in Figure 2.

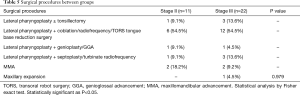
One-hundred and six patients underwent surgery after DISE and 33 patients had a post-surgical sleep study between 3 to 6 months that were available at the time of this study. No significant difference was found in baseline characteristics, pre- and post-surgical sleep study outcomes, surgical response rate between groups (Table 4). The same can be seen in relation the surgical procedures performed across groups (Table 5).
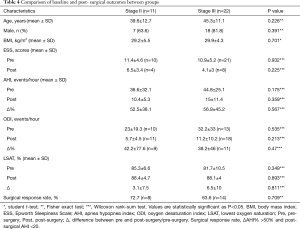
Full table
Full table
Discussion
In this study, DISE was used to explore the UA obstructive features in Friedman's stages II and III. To our knowledge, no articles were published to analyze the correlation between DISE findings and Friedman staging. The main finding in this study was that stage III patients had a significantly higher proportion of more than 2 obstructive sites when compared to stage II patients. Most patients presented upper and lower level combined obstruction in both stage II and III, V + O + T was the most verified association. Surgical procedures based on DISE assessment could obtain similar success rate in both stage III and stage II patients.
In general, most Friedman II and III stage subjects presented multisites UA obstruction (91.8% in stage II and 97.1% in stage III), combined obstructions of palatopharynx and hypopharynx (71% and 81%, respectively), but less isolated hypopharyngeal obstruction (4% and 6%, respectively). As comparison, the percentage of multiple sites obstruction and isolated hypopharyngeal obstruction was 68.2% (37) and 11% (38) in broad sleep-disordered breathing population, respectively. DISE provided new evidence about collapse characteristics in those subjects, who were more susceptible to multi-site combined collapse and obstruction. Meanwhile, velum was still the most common collapse site with 94.5% and 96.1% in stages II and III, respectively.
The main difference between stages was that stage III subjects were more susceptible to more than two obstructive sites, with increased severity of hypopharyngeal obstruction, contributing to lower success rates than stage II. Meanwhile, there was a higher proportion of lateral wall narrowing in stage II (67.1%) and III (79.4%) compared to the general OSA population (35%) (39). This likely contributes to a lower surgical success rate to soft tissue procedures in this sub-population by non skeletal procedures (40,41), however surgical responses and results may be better through maxillomandibular advancement (MMA) (42). In our experience, simultaneous hypopharyngeal procedures are considered based on DISE findings to improve surgical results. MMA was considered thoughtfully if hypopharyngeal lateral wall collapse is observed.
Some studies concluded that it was unnecessary to perform DISE since there would be no significant outcome difference between all-sites and partial-sites surgical intervention (43,44), but DISE revealed more multiple sites obstruction and pharyngeal lateral wall collapse in high AHI patients (37,45,46). Spector et al. found 70% success rate in stage II and 66% in stage III through multilevel surgeries by DISE directions (47).
Other studies did not find the relationship between Friedman stage and incidence of retroglossal collapse (48,49). Similarly, the association between high FTP and tongue base obstruction was not found either (50,51). Our results showed that either retroglossal or retropalatal, oropharyngeal and epiglottal collapse had no significant association with stages II or III. Therefore, while Friedman staging may be a primary method for judging the severity of collapsibility, DISE provides additional evaluation with more information identifying the obstructive characteristics of the patient with OSA.
One limitation of this study was that most subjects of stage II were classified as IIa (86.3%). In general, patients with tonsils 3 or 4 were seldom performed DISE prior to surgery, according to the protocol used by our department. It is known that cases with higher amygdala grade in general have a positive correlation with higher AHI and higher success rate (52-54). In addition, the subjects who were from sleep clinic and CPAP intolerant and sought for possible surgical therapy may have increased our selection bias. Besides that, DISE findings also altered with type (55), depth (56) and duration of sedation (57), the protocol that we used only applied dexmedetomidine, thus not allowing comparison with other studies using different drugs.
Conclusions
Upper and lower combined obstruction was the main collapse characteristic in both groups. Friedman stage III subjects had more than 2 sites of obstruction when compared to stage II patients. No differences were identified for the degree and form of obstruction between stages II and III.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1471
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1471). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the Institutional Review Board and Hospital Research Ethics Committee of Stanford Hospital and Clinics (approval number 35054). All patients enrolled completed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torres G, Sánchez-de-la-Torre M, Barbé F. Relationship between OSA and hypertension. Chest 2015;148:824-32. [Crossref] [PubMed]
- Jennum P, Tønnesen P, Ibsen R, et al. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med 2017;36:62-6. [Crossref] [PubMed]
- Alexiev F, Brill AK, Ott SR, et al. Sleep-disordered breathing and stroke: chicken or egg? J Thorac Dis 2018;10:S4244-52. [Crossref] [PubMed]
- Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med 2013;1:329-38. [Crossref] [PubMed]
- Kendzerska T, Gershon AS, Hawker G, et al. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med 2014;11:e1001599. [Crossref] [PubMed]
- Alberto EC, Tanigawa T, Maruyama K, et al. Relationships between nocturnal intermittent hypoxia, arterial stiffness and cardiovascular risk factors in a community-based population: the Toon Health Study. J Atheroscler Thromb 2014;21:1290-7. [Crossref] [PubMed]
- Trzepizur W, Le Vaillant M, Meslier N, et al. Independent association between nocturnal intermittent hypoxemia and metabolic dyslipidemia. Chest 2013;143:1584-9. [Crossref] [PubMed]
- Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med 2019;380:1442-9. [Crossref] [PubMed]
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:136-43. [Crossref] [PubMed]
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. [Crossref] [PubMed]
- Walia HK, Thompson NR, Strohl KP, et al. Upper Airway Stimulation vs Positive Airway Pressure Impact on BP and Sleepiness Symptoms in OSA. Chest 2020;157:173-83. [Crossref] [PubMed]
- Freedman N. Treatment of obstructive sleep apnea: choosing the best positive airway pressure device. Sleep Med Clin 2020;15:205-18. [Crossref] [PubMed]
- Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep 2011;34:111-9. [Crossref] [PubMed]
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 2008;5:173-8. [Crossref] [PubMed]
- Ikematsu T. Study of snoring, fourth report. Ther J Jpn Otorhinolaryngol 1964;64:434-5.
- Fujita S, Conway W, Zurick F, et al. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981;89:923-34. [Crossref] [PubMed]
- Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996;19:156-77. [Crossref] [PubMed]
- Senior BA, Rosenthal L, Lumley A, et al. Efficacy of uvulopalatopharyngoplasty in unselected patients with mild obstructive sleep apnea. Otolaryngol Head Neck Surg 2000;123:179-82. [Crossref] [PubMed]
- Sher AE. Update on upper airway surgery for obstructive sleep apnea. Curr Opin Pulm Med 1995;1:504-11. [Crossref] [PubMed]
- Farmer WC, Giudici SC. Site of airway collapse in obstructive sleep apnea after uvulopalatopharyngoplasty. Ann Otol Rhinol Laryngol 2000;109:581-4. [Crossref] [PubMed]
- Kezirian EJ. Nonresponders to pharyngeal surgery for obstructive sleep apnea: insights from drug-induced sleep endoscopy. Laryngoscope 2011;121:1320-6. [Crossref] [PubMed]
- Friedman M, Tanyeri H, LaRosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope 1999;109:1901-7. [Crossref] [PubMed]
- Friedman M, Ibrahim H, Joseph NJ. Staging of obstructive sleep apnea/hypopnea syndrome: a guide to appropriate treatment. Laryngoscope 2004;114:454-9. [Crossref] [PubMed]
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep disordered breathing. Otolaryngol Head Neck Surg 2002;127:13-21. [Crossref] [PubMed]
- Li HY, Wang PC, Lee LA, et al. Prediction of uvulopalatopharyngoplasty outcome: anatomy-based staging system versus severity-based staging system. Sleep 2006;29:1537-41. [Crossref] [PubMed]
- Rodrigues MM, Real Gabrielli MF, Watanabe ER, et al. Correlation between the Friedman Staging System and the upper airway volume in patients with obstructive sleep apnea. J Oral Maxillofac Surg 2015;73:162-7. [Crossref] [PubMed]
- Aktas O, Erdur O, Cirik AA, et al. The role of drug-induced sleep endoscopy in surgical planning for obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 2015;272:2039-43. [Crossref] [PubMed]
- Rodriguez-Bruno K, Goldberg AN, McCulloch CE, et al. Test-retest reliability of drug-induced sleep endoscopy. Otolaryngol Head Neck Surg 2009;140:646-51. [Crossref] [PubMed]
- Kezirian EJ, White DP, Malhotra A, et al. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg 2010;136:393-7. [Crossref] [PubMed]
- Vroegop AV, Vanderveken OM, Wouters K, et al. Observer variation in drug-induced sleep endoscopy: experienced versus nonexperienced ear, nose, and throat surgeons. Sleep 2013;36:947-53. [Crossref] [PubMed]
- Fernández-Julián E, García-Pérez MÁ, García-Callejo J, et al. Surgical planning after sleep versus awake techniques in patients with obstructive sleep apnea. Laryngoscope 2014;124:1970-4. [Crossref] [PubMed]
- Eichler C, Sommer JU, Stuck BA, et al. Does drug-induced sleep endoscopy change the treatment concept of patients with snoring and obstructive sleep apnea? Sleep Breath 2013;17:63-8. [Crossref] [PubMed]
- Koutsourelakis I, Safiruddin F, Ravesloot M, et al. Surgery for obstructive sleep apnea: sleep endoscopy determinants of outcome. Laryngoscope 2012;122:2587-91. [Crossref] [PubMed]
- Capasso R, Rosa T, Tsou DY, et al. Variable findings for drug-induced sleep endoscopy in obstructive sleep apnea with propofol versus dexmedetomidine. Otolaryngol Head Neck Surg 2016;154:765-70. [Crossref] [PubMed]
- De Vito A, Carrasco Llatas M, Vanni A, et al. European position paper on drug-induced sedation endoscopy (DISE). Sleep Breath 2014;18:453-65. [Crossref] [PubMed]
- Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol 2011;268:1233-6. [Crossref] [PubMed]
- Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope 2014;124:797-802. [Crossref] [PubMed]
- Gillespie MB, Reddy RP, White DR, et al. A trial of drug-induced sleep endoscopy in the surgical management of sleep-disordered breathing. Laryngoscope 2013;123:277-82. [Crossref] [PubMed]
- Woodson BT. A method to describe the pharyngeal airway. Laryngoscope 2015;125:1233-8. [Crossref] [PubMed]
- Iwanaga K, Hasegawa K, Shibata N, et al. Endoscopic examination of obstructive sleep apnea syndrome patients during drug-induced sleep. Acta Otolaryngol Suppl 2003;550:36-40. [Crossref] [PubMed]
- Soares D, Sinawe H, Folbe AJ, et al. Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep apnea surgery. Laryngoscope 2012;122:473-9. [Crossref] [PubMed]
- Liu SY, Huon LK, Powell NB, et al. Lateral pharyngeal wall tension after maxillomandibular advancement for obstructive sleep apnea is a marker for surgical success: observations from drug-induced sleep endoscopy. J Oral Maxillofac Surg 2015;73:1575-82. [Crossref] [PubMed]
- Blumen MB, Latournerie V, Bequignon E, et al. Are the obstruction sites visualized on drug-induced sleep endoscopy reliable? Sleep Breath 2015;19:1021-6. [Crossref] [PubMed]
- Golbin D, Musgrave B, Succar E, et al. Clinical analysis of drug-induced sleep endoscopy for the OSA patient. Laryngoscope 2016;126:249-53. [Crossref] [PubMed]
- Koo SK, Choi JW, Myung NS, et al. Analysis of obstruction site in obstructive sleep apnea syndrome patients by drug induced sleep endoscopy. Am J Otolaryngol 2013;34:626-30. [Crossref] [PubMed]
- Huon LK, Liu SY, Shih TT, et al. Dynamic upper airway collapse observed from sleep MRI: BMI-matched severe and mild OSA patients. Eur Arch Otorhinolaryngol 2016;273:4021-6. [Crossref] [PubMed]
- Spector ME, Glazer TA, Hoff PT. Addressing the retrolingual space in obstructive sleep apnea: outcomes stratified by Friedman stage in patients undergoing transoral robotic surgery. ORL J Otorhinolaryngol Relat Spec 2016;78:1-8. [Crossref] [PubMed]
- Zerpa Zerpa V, Carrasco Llatas M, Agostini Porras G, et al. Drug-induced sedation endoscopy versus clinical exploration for the diagnosis of severe upper airway obstruction in OSAHS patients. Sleep Breath 2015;19:1367-72. [Crossref] [PubMed]
- Lee CH, Won TB, Cha W, et al. Obstructive site localization using multisensor manometry versus the Friedman staging system in obstructive sleep apnea. Eur Arch Otorhinolaryngol 2008;265:171-7. [Crossref] [PubMed]
- den Herder C, van Tinteren H, de Vries N. Sleep endoscopy versus modified Mallampati score in sleep apnea and snoring. Laryngoscope 2005;115:735-9. [Crossref] [PubMed]
- Soares D, Folbe AJ, Yoo G, et al. Drug-induced sleep endoscopy vs awake Müller’s maneuver in the diagnosis of severe upper airway obstruction. Otolaryngol Head Neck Surg 2013;148:151-6. [Crossref] [PubMed]
- Jara SM, Weaver EM. Association of palatine tonsil size and obstructive sleep apnea in adults. Laryngoscope 2018;128:1002-6. [Crossref] [PubMed]
- Camacho M, Li D, Kawai M, et al. Tonsillectomy for adult obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope 2016;126:2176-86. [Crossref] [PubMed]
- Smith MM, Peterson E, Yaremchuk KL. The role of tonsillectomy in adults with tonsillar hypertrophy and obstructive sleep apnea. Otolaryngol Head Neck Surg 2017;157:331-5. [Crossref] [PubMed]
- Viana A, Zhao C, Rosa T, et al. The effect of sedating agents on drug-induced sleep endoscopy findings. Laryngoscope 2019;129:506-13. [Crossref] [PubMed]
- Hong SD, Dhong HJ, Kim HY, et al. Change of obstruction level during drug-induced sleep endoscopy according to sedation depth in obstructive sleep apnea. Laryngoscope 2013;123:2896-9. [Crossref] [PubMed]
- Heo SJ, Park CM, Kim JS. Time-dependent changes in the obstruction pattern during drug-induced sleep endoscopy. Am J Otolaryngol 2014;35:42-7. [Crossref] [PubMed]


