
Impact of da Vinci Xi robot in pulmonary resection
Introduction
Minimally invasive surgery using a video-assisted thoracoscopic surgery (VATS) technique has emerged as the standard surgical approach for lung resections due to reduced post-operative pain (1-3), fewer overall complications (4-6), shorter length of stay (2,7), lower cost (6), and better long-term survival (2,8) compared to open thoracotomy. Although VATS pulmonary resections have superior outcomes compared to open pulmonary resections, VATS is limited to early stage cancer or more advanced cancer with certain anatomic features (9-11) for most surgeons, mainly due to the limitations of instruments within the chest cavity.
The introduction of computer-aided robotic-assisted surgery in the year 2000 overcame some technical challenges associated with VATS and provided additional tools for performing minimally invasive surgery (12). Robotic-assisted surgery has several advantages, including three-dimensional binocular vision, elimination of the natural surgeon tremor, increased degree of motion, enhanced manipulation with wristed instruments, and improved dexterity over conventional VATS (13). However, an initial robot platform (da Vinci S/Si, Intuitive Surgical, Sunnyvale, CA, USA, Si robot) was created to perform operations that did not require a division of vascular structure using the robot. Thus, the initial use of the robot technology in anatomic pulmonary resections required bedside assistance to perform the dividing of vascular structures using a handheld vascular stapler (14). For this version of the robot, studies showed that patients treated with a robotic Si approach had a lower morbidity and mortality than patients undergoing open thoracotomy (15). However, the Si robot system had outcomes comparable to VATS pulmonary resection (16).
In 2016, there was significant improvement in robot systems, namely the addition of a vascular robot stapler (da Vinci Xi, Intuitive Surgical, Sunnyvale, CA, USA, Xi robot), as well as a new design that allowed the robot to move to the patient’s position (rather than the patient moving to the robot) and the Xi robot had all of the tools to perform robotic pulmonary resection with minimal help from an assistant. In our initial experience with the Xi robot, we have shown that we can decrease the rate of conversion to thoracotomy compared to VATS pulmonary resection (17). The aim of the present study was to determine the impact of the da Vinci Xi robot in pulmonary resection outcomes in a single institution thoracic surgery program. We present the following article in accordance with the STROBE reporting checklist (18) (available at http://dx.doi.org/10.21037/jtd-20-720).
Methods
At Houston Methodist Hospital we performed a retrospective analysis of prospectively collected Society of Thoracic Surgeons (STS) data. The Institutional Review Board at Houston Methodist Research Institute approved the study (Pro00013680 and Pro00013298) and informed consent was obtained from patient from 2016–2019 and patients from 2012–2015 consents were waived since this study was determined to be minimal risk to patients. The study conformed to the provisions of the Declaration of Helsinki. The database was queried for consecutive patients who underwent elective pulmonary resections at Houston Methodist Hospital performed by surgeons in the Division of Thoracic Surgery from 2012 to 2019. All patients who underwent emergent pulmonary resection and patients who underwent pulmonary resection as part of a two-step procedure for cardiac sarcoma with pulmonary involvement were excluded. Additionally, patient demographics, clinic-pathologic features, operative approach, and surgical outcomes were evaluated.
From the STS database, clinical characteristics including age, gender, body mass index (BMI), smoking history, history of preoperative chemotherapy or thoracic radiation therapy, American Society of Anesthesiologists (ASA) physical status class, and the presence of co-morbidities such as hypertension, congestive heart failure, coronary artery disease, diabetes, interstitial fibrosis, end stage renal disease, and cerebrovascular accidents were obtained. From the STS database and patients’ electronic health records, the etiology of the lung disease and the history of any prior thoracic surgeries were ascertained, as well as operative details such as the type of procedure, the surgical approach (including open, VATS, and robotic approaches), the type of robotic platform (Si or Xi), the duration of the procedure, any postoperative complications, major postoperative complications (air leak >5 days, surgical site infection, pulmonary emboli, atrial arrhythmia, ventricular arrhythmia, pneumonia, myocardial infarct, empyema, bronchopleural fistula, respiratory failure, adult respiratory distress syndrome, tracheostomy, renal failure, sepsis, DVT, stroke and unexpected return to OR) any requirements for blood transfusion, the hospital length of stay (LOS), mortality, any readmissions within 30 days of discharge, and any pathology information, such as the pathologic lung cancer stage and the number of retrieved lymph nodes. Characteristics of the surgeon who performed the pulmonary resection were analyzed. The surgeon’s overall experience in thoracic surgical procedures was categorized by the number of years of practice since fellowship training either ≤5 or >5 years.
The cohort was divided into three time periods: (I) prior to introduction of a robot with a vascular stapler or da Vinci Xi robot, predominantly VATS; (II) initial robot experience, comprising the first 18 months after the introduction of da Vinci Xi robot; and (III) mature robot experience, covering the next 18 months. Patient characteristics and outcomes at different time periods in the learning curve were analyzed and the impact of the addition of the da Vinci Xi robot on the surgical outcomes was evaluated. Univariate and multivariate logistic regression modeling was performed to determine the factors associated with complications and outcomes after surgery.
Demographic and clinical data were reported as frequencies and proportions for categorical variables and as median and interquartile ranges (IQR) or means (± standard deviation, SD) for continuous variables as appropriate. Differences between groups were compared using the Chi-square or Fisher’s exact tests for categorical variables and an unpaired t-test, ANOVA or Kruskal Wallis tests for continuous variables as appropriate. Univariate and multivariate analysis was then performed to determine the factors associated with post-surgical complications. Univariate logistic regression analysis and multiple logistic regression modeling was also performed to determine the characteristics associated with the outcomes. Variables having a P value of <0.2 in the univariate analysis or considered clinically significant were investigated further by multiple logistic regression modeling. The Likelihood Ratio test was used to reduce the model subsets. The best model was selected based on the smallest Bayesian information criterion (BIC). The area under the Receiver Operating Characteristic (ROC) curve (AUC) determined model discrimination. Model calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test, with a non-significant P value indicating good calibration.
Propensity score match between the two groups—VATS vs. Xi robot anatomic pulmonary resections was also analyzed, based on a set of covariates including age, gender, body mass index, ASA classification, category of disease, forced expiratory volume in 1 second (FEV1), carbon monoxide lung diffusion capacity (DLCO); in the case of lung cancer, and lung cancer staging. Multiple logistic regression modeling was then performed to determine if the Xi robot was associated with a decrease in complications and outcomes in the matched cohort. All analyses and propensity score matching were performed using Stata MP version 16.1 (StataCorp LLC, College Station, TX, USA). A P value of <0.05 was considered statistically significant.
Results
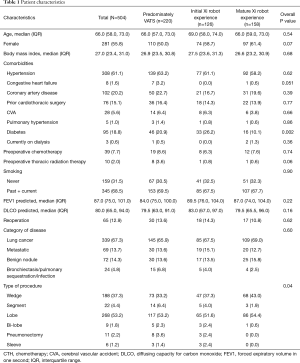
There were 504 patients who met the inclusion criteria from 2012 to 2019. Two hundred twenty (220) patients (43.7%) fell in the first time period (predominately VATS, prior to the use of the da Vinci Xi robot); 126 patient-procedures (25%) occurred in the second time period (the initial robot experience); and 158 patient-procedures (31.1%) were identified in the third time period (the mature robot experience, Table 1). During the predominately VATS time period, 19 surgeries (8.6%) were started with a thoracotomy, 7 (3.2%) were performed minimally invasively with the robot without a vascular stapler (Si robot), and 194 (88.2%) were performed with VATS. However, after the introduction of the robot with a vascular stapler (Xi robot) during the second time period, there were 2 patient-surgeries (1.2%) performed with an open thoracotomy, 84 (66.7%) performed with the Xi robot, and 40 (31.7%) performed with VATS. Furthermore, during the third time period, there were no patient-surgeries started with thoracotomy; 139 (88%) were performed with the Xi robot, and only 19 (12%) were performed with VATS.
Full table
The median age for our cohort was 66 (IQR 58, 73) years; 44.2% were men, and the median BMI was 27 (IQR 23.4, 31), with 67.3% of patients having lung cancer and 13.7% having metastatic disease in the lung. There were no significant differences between the three groups in terms of age, sex, BMI, co-morbidities (hypertension, coronary artery disease, congestive heart failure, pulmonary hypertension, interstitial lung disease, and cerebrovascular accidents), smoking history, category of primary disease, and number of patients with prior thoracic surgeries. There was higher number of diabetic patients in the initial robotic group compared to the VATS and mature robotic groups (26.2%, 20.9%, and 10.1%, respectively, P=0.002) as well as difference in type of procedure (Table 1).
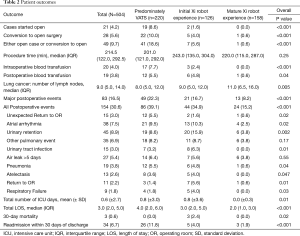
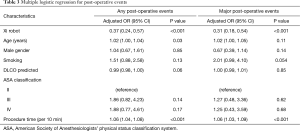
There were a higher number of surgeries that converted to open procedures in the predominantly VATS group compared to the initial and mature robotic groups: 22 cases (10%), 5 cases (4%), and 1 case (0.6%), respectively (P<0.001). Additionally, there were significantly less major postoperative complications (8.2% vs. 16.7% vs. 22.3%, P<0.001, Figure 1A), less post-operative complications (15.2% vs. 34.9% vs. 39.1%, P<0.001), fewer unexpected returns to the operating room (0.6% vs. 1.6% vs. 5.5%, P=002), shorter median length of stays (2 vs. 3 vs. 4 days, P<0.001, Figure 1B), and lower readmission rates (1.9% vs. 4% vs. 11.8%, P<0.001, Figure 1C) in the mature robot period compared to the initial robot period and the predominately VATS period, respectively. Moreover, there was a significantly higher number of lymph node retrievals in the mature robotic period than in the initial robotic or predominantly VATS time periods [median (IQR), 11 (6, 16), 9 (5, 12), and 8 (5, 12), respectively, P=0.005] (Table 2). Multivariate analysis showed that the Xi robot (OR 0.37; 95% CI, 0.24–0.57, P<0.001) was associated with a decrease in post-operative events, while age (OR 1.02; 95% CI, 1.00–1.04, P=0.03) and procedure time (OR 1.06; 95% CI, 1.04–1.08, P<0.001) were associated with an increase in post-operative events (Table 3). Furthermore, multivariate analysis showed that the Xi robot (OR 0.31; 95% CI, 0.18–0.54, P<0.001) was associated with a decrease in major post-operative events, while procedure time (OR 1.06; 95% CI, 1.03–1.09, P<0.001) was associated with increase in major post-operative events (Table 3).

Full table
Full table
During the three time periods, there was a total of five surgeons in the practice who performed pulmonary resections. One of the senior surgeons left the practice at the end of the predominately VATS time period and one junior surgeon left at the end of the initial robot time period. During each time period, one junior surgeon was hired from fellowship training. The change in compliment of surgeons led to each time period having one surgeon with >5 years of experience and two surgeons having≤ 5 years of experience since fellowship training. At the beginning of the initial robot experience, none of the surgeons had any experience with da Vinci Xi robot and one surgeon had performed <10 pulmonary resections using da Vinci Si robot.
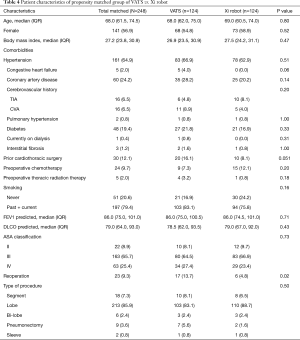
Next, we performed propensity-matched analysis of patients who underwent anatomic pulmonary resection with either VATS (n=124) or the Xi robot (n=124). There were no significant differences in age, gender, body mass index, comorbidities, prior cardiothoracic surgery, pre-operative chemotherapy, pre-operative radiation therapy, smoking history, FEV1, DLCO, ASA classification, reoperation rate and type of procedure (Table 4). Compared to the VATS group, the Xi robot group had significantly fewer patients who converted to open thoracotomies (2.4% vs. 15.3%, P<0.001, Table 5), significantly less requirements for intraoperative blood transfusions (2.4% vs. 9.7%, P=0.02), significantly lower overall postoperative events (32.3% vs. 49.2%, P=0.01), lower major postoperative events (15.3% vs. 29.8%, Figure 2A, Table 5), significantly shorter ICU admissions (mean ± SD, 0.3±1.1 vs. 1.1±3.8, P=0.02), significantly less LOS [median (IQR) 3.0 (2.0, 5.0) vs. 4.5 (3.0, 6.0), P<0.001, Figure 2B], and lower frequency of readmissions (0.8% vs. 10.5%, P<0.001, Figure 2C, Table 5). However, the Xi robot group had significantly higher median number of lymph node harvested during the operation compared to VATS group (12 vs. 9, P=0.001). The multivariate analysis for postoperative event showed robotic lung resection (OR 0.51; 95% CI, 0.29–0.91, P=0.02) was still associated with fewer postoperative complications, while age (OR 1.03; 95% CI, 1.00–1.06, P=0.03) and smoking (OR 2.80; 95% CI, 1.29–6.09, P=0.01) were associated with increase in all post-operative complications (Table 6). Furthermore, the multivariate analysis for major post-operative events showed Xi robotic lung resection (OR 0.42; 95% CI, 0.21–-0.82, P=0.01) was associated with fewer major post-operative events, while smoking (OR 7.62; 95% CI, 1.76–32.97, P=0.01) was associated with increase in major post-operative complication (Table 6).
Full table
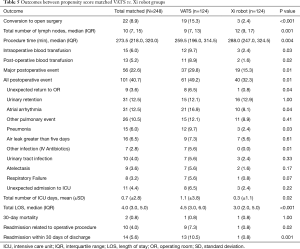
Full table


Full table
Discussion
In our practice, we had difficulty adopting the Si robot platform to perform pulmonary resections mainly because we did not have skilled surgical assistants to support the surgeons with performing complex operations. At the time when the Si robot was introduced, we had rotating general surgery residents who had no experience or only one month of experience with minimally invasive pulmonary resections. We were able to perform VATS pulmonary resections because surgeons could easily direct the inexperienced assistant working across the patient’s bedside. When the Si robot was used however, it was challenging for surgeons to direct an unskilled assistant, and it was not safe to have an inexperienced assistant position the stapler around a pulmonary vessel and then fire the stapler. These limitations prevented us from adopting the Si robot into our practice for pulmonary resections.
The Xi robot platform possessed many advantages over the Si robot platform, including the ability for surgeons to use a vascular stapler to divide vascular structures, the ability to reposition the camera in any port (19), and the ability of the robot to adjust to the patient’s position rather than the patient moving to the robot. We adopted a standard port placement and dissection technique using the Xi robot and mastered the technique over time (20). This standard technique allowed us to gain further experience in complex operations and overcome the limitation of needing a skilled bedside assistant to manipulate the lung(s) for exposure, to suction blood, or to use the stapler. The ability to control a camera along with three other instruments has allowed the surgeon to have complete control during the conduct of the operation, translating to less variability in the operating room, which in turn leads to consistent surgical outcomes. In this study, we were able to show that surgeons gaining experience with the capabilities of the Xi robot contributed to improved outcomes. The introduction of the Xi robot when we performed predominately VATS pulmonary resections initially led to a decrease in thoracotomies (17). As the surgeons gained more experience with the Xi robot, we saw a decrease in complication and readmission rates, as well as in length of stay.
The significant decrease in complication rates were also found after performing propensity matching between VATS and robot-assisted anatomic pulmonary resections. VATS has emerged as a safe and feasible alternative to traditional open thoracotomy for lung resections, with better short- and long-term outcomes. Evidence-based therapeutic guidelines have suggested the VATS approach for anatomical lung resections in non-small cell lung cancer patients, whenever possible (21). However, VATS has many limitations that impede its ability to perform complex lung resection procedures, such as tremor amplification, as well as the instruments’ fulcrum effect. Furthermore, VATS requires substantial training and has a longer learning curve. The improved outcomes of Xi robot in pulmonary resection was found despite having a very junior complement of surgeons. During the adoption of the robot, none of the surgeons had previous experience with robot-assisted pulmonary resection, and two of the surgeons just started their clinical practice.
In the current study, we have shown that the use of the Xi robot in lung resection procedures was associated with lower morbidity rates, shorter LOS, and fewer readmissions compared to VATS. The main difference between our study and previous studies comparing the robotic and VATS approaches is the usage of the Xi robot with a vascular stapler instead of the Si robot with bedside assistant(s) using a handheld robotic stapler. Other studies may not have shown a benefit due to the use of earlier-generation robotic platforms (da Vinci Si) (16). Liang et al. conducted a meta-analysis to compare robotic vs. VATS in anatomical lung resections for cancer; this analysis included 14 studies using the da Vinci Si, and concluded that there was decreased mortality and conversions to thoracotomies in the robotic arm, but comparable morbidity and LOS between both groups (22). Emmert et al. conducted a meta-analysis of 10 studies that compared robotic assisted and VATS lung resections; this analysis found decreased mortality in the robotic arm with comparable LOS between both groups (23). On the other hand, in a recent comparative study between Si robot and VATS-assisted lung resections, Huang et al. concluded that the Si robot was associated with prolonged LOS and comparable survival outcomes with VATS (24). All of these studies suggest that the Si robot may not reliably reduce conversions to thoracotomy and may not lead to improved LOS or complications. Yet, the previous studies comparing postoperative complications between robot-assisted and VATS-assisted lung resections may have lacked the experience necessary to detect a difference in outcome. We did not find significant improvement in outcomes during our initial experience with the Xi robot (17); however, once we gained enough experience with a standardized method for performing pulmonary resections with enough patient-procedures, we saw increased benefits of the Xi robot platform.
Many studies have reported the numbers of lymph nodes (LNs) harvested using different approaches in lung resections for cancer. Results from randomized controlled trials conducted by the American College of Surgery Oncology Group showed no difference in the number of LNs retrieved by VATS compared to the number by open thoracotomy (25). Other studies have shown no differences in the numbers of LNs removed by robot-assisted procedures compared to VATS (22,24,26). However, our results showed a greater number of LNs harvested by robotic approaches in comparison to VATS approaches; this finding is consistent with prior publications that showed the superiority of robotic approaches in the number of retrieved LNs (27). Our ability to harvest lymph nodes also improved over time with the highest number of lymph node being harvested during the mature robot period.
We recognize the main limitation of our study is the retrospective nature of the study and the inherent bias associated with retrospective studies. In addition, since this is not a randomized controlled trial, the factors such as ICU stay and length of hospital stay could have been influenced by the primary surgeon taking care of the patient as well as other non-surgical factors. The strength of our study lies in our analysis of prospectively collected data from the Society of Thoracic Surgeons database at Houston Methodist Hospital. A designated data abstractor collected the demographics and the operation and postoperative complications in real time. Having a prospective collection of data provides an accurate assessment of past events and outcomes. However, we also performed propensity score matching based on well-characterized clinical and pathological data via the detailed STS registry to decrease selection bias among groups. Despite these challenges, we feel that the sample size and propensity matching represent a true phenomenon. Further long-term outcomes research and randomized controlled trials to compare robotic-assisted and VATS approaches would further elucidate our findings.
Adopting the da Vinci Xi robot in our institution was associated with a significant decrease in post-operative complications, length of stay, and re-admission rates when compared to VATS pulmonary resections. The benefits of the Xi robot platform improved as surgeons gained more experience. Randomized, well-designed, controlled trials are still needed, however, to determine the short- and long-term benefits of using computer-aided robots in pulmonary resection surgeries.
Acknowledgments
We thank Mark Celeste for language editing of this manuscript. We thank Kathryn J. Schulze and Debra Selig-Rosen for providing the data collected for the Society of Thoracic Surgeons at Houston Methodist Hospital.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-720
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-720
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-720). EY Chan reports personal fees from Veran, outside the submitted work; MP Kim reports personal fees from Veran, personal fees from Intuitive Surgical, personal fees from Medtronic, outside the submitted work; MP Kim also serves as an unpaid editorial board member of Journal of Thoracic Disease from Sep 2018 to Aug 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). The Institutional Review Board at Houston Methodist Research Institute approved the study (Pro00013680 and Pro00013298) and informed consent was obtained from patient from 2016-2019 and patients from 2012-2015 consents were waived since this study was determined to be minimal risk to patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Alam N, Flores RM. Video-assisted thoracic surgery (VATS) lobectomy: the evidence base. JSLS 2007;11:368-74. [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiovasc Surg 1992;104:1679-85; discussion 1685-7.
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Sugiura H, Morikawa T, Kaji M, et al. Long-term benefits for the quality of life after video-assisted thoracoscopic lobectomy in patients with lung cancer. Surg Laparosc Endosc Percutan Tech 1999;9:403-8. [Crossref] [PubMed]
- Samson P, Guitron J, Reed MF, et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013;145:1512-8. [Crossref] [PubMed]
- Zhao H, Bu L, Yang F, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer: the learning curve. World J Surg 2010;34:2368-72. [Crossref] [PubMed]
- Marty-Ane CH, Canaud L, Solovei L, et al. Video-assisted thoracoscopic lobectomy: an unavoidable trend? A retrospective single-institution series of 410 cases. Interact Cardiovasc Thorac Surg 2013;17:36-43. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 2016;101:28-34. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Robotic-assisted pulmonary resection - Right upper lobectomy. Ann Cardiothorac Surg 2012;1:77-85. [PubMed]
- Zhang L, Gao S. Robot-assisted thoracic surgery versus open thoracic surgery for lung cancer: a system review and meta-analysis. Int J Clin Exp Med 2015;8:17804-10. [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Kim MP, Nguyen DT, Meisenbach LM, et al. Da Vinci Xi robot decreases the number of thoracotomy cases in pulmonary resection. J Thorac Dis 2019;11:145-53. [Crossref] [PubMed]
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [Crossref] [PubMed]
- Morelli L, Di Franco G, Lorenzoni V, et al. Structured cost analysis of robotic TME resection for rectal cancer: a comparison between the da Vinci Si and Xi in a single surgeon's experience. Surg Endosc 2019;33:1858-69. [Crossref] [PubMed]
- Kim MP, Chan EY. "Five on a dice" port placement for robot-assisted thoracoscopic right upper lobectomy using robotic stapler. J Thorac Dis 2017;9:5355-62. [Crossref] [PubMed]
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]
- Emmert A, Straube C, Buentzel J, et al. Robotic versus thoracoscopic lung resection: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7633. [Crossref] [PubMed]
- Huang L, Shen Y, Onaitis M. Comparative study of anatomic lung resection by robotic vs. video-assisted thoracoscopic surgery. J Thorac Dis 2019;11:1243-50. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Bao F, Zhang C, Yang Y, et al. Comparison of robotic and video-assisted thoracic surgery for lung cancer: a propensity-matched analysis. J Thorac Dis 2016;8:1798-803. [Crossref] [PubMed]
- Velez-Cubian FO, Rodriguez KL, Thau MR, et al. Efficacy of lymph node dissection during robotic-assisted lobectomy for non-small cell lung cancer: retrospective review of 159 consecutive cases. J Thorac Dis 2016;8:2454-63. [Crossref] [PubMed]


