Tuberculous pleural effusions: advances and controversies
Introduction
Mycobacterium tuberculosis (M. tuberculosis) is a pathogenic bacterial species in the family Mycobacteriaceae and the causative agent of most cases of tuberculosis (TB) (1). Despite being isolated by Robert Koch in 1882, as well as the availability of effective treatment and the use of a live attenuated vaccine in many parts of the world, TB remains one of the deadliest communicable diseases. In 2013, an estimated 9 million people developed active TB, with 1.5 million deaths attributed to the disease (2). According to the World Health Organisation the incidence of pulmonary TB in some regions is as high as 1,000 cases per 100,000 persons (2). Although TB affects the lungs in the majority of patients, extrapulmonary TB serves as the initial presentation in about 25% of adults, and primarily involves the lymph nodes and pleura (3). This review gives an overview of the pathogenesis, clinical presentation, diagnosis and treatment of TB pleural effusions, highlighting recent advances and controversies.
Pathogenesis
Until recently TB pleural effusions were thought to occur largely as a result of a delayed hypersensitivity reaction. Injecting tuberculin into the pleural cavity of guinea-pigs sensitized with heat killed M. tuberculosis, produces a large protein-rich pleural effusion over a 24-hour period, which is completely suppressed by antilymphocyte serum (4). Based on this model and the fact that researchers were unable to culture M. tuberculosis from pleural fluids, the pathogenesis was presumed to be due to delayed hypersensitivity rather than a direct infection of the pleural space. With the advent of improved culture media it is now possible to culture M. tuberculosis from both pleural fluid and pleural tissue in as many as 70% of cases (5), and following Koch’s postulate for infection, this would suggest a causal relationship (6).
The pleural effusion is likely a manifestation of paucibacillary mycobacterial infection within the pleural space, which is acquired from initial parenchymal lesions and results in an immunological response that both increases pleural fluid formation and decreases pleural fluid removal (7). Initially, there is a rapid neutrophilic inflammatory response within the pleura which is symptomatic. This is followed by a protracted lymphocyte driven immune reaction which is accompanied by pleural granuloma formation and release of adenosine deaminase (ADA). It is therefore plausible that the likelihood of a positive pleural fluid culture decreases with time, as the effusion becomes lymphocyte predominant, and viable mycobacteria are contained.
Similar to lung parenchymal TB, the pathogenetic hypothesis of pleural TB suggests that a strong T-helper type 1 (Th1)-like immunity (interferon dominant) is essential for the containment of M. tuberculosis, while these protective effects are antagonized by T-helper type 2 cytokines, primarily interleukin (IL)-4 (8). Activated CD3+ and CD4+ Th1 cells, through the release of interferon gamma (IFN-γ) and other Th1 cytokines, activate macrophages to kill M. tuberculosis, whereas Th2 cytokines may antagonize this effect (9). The predominance of Th1 immunity in TB pleural effusions is confirmed by the high levels of IFN-γ, and other inflammatory cytokines (e.g., IL 12), while the proportion of helper T-cells in pleural fluid are also elevated compared with serum or peripheral blood (8,10,11), thus creating a compartmentalised pleural space. The frequency of IL-4 producing T-cells, representing Th2 immunity, is significantly lower in pleural fluid compared to peripheral blood (8). This compartmentalisation may not occur immediately after infection, as shown by animal and in vitro studies (12,13). Polymorphonuclear leukocytes are the first cells to respond, remaining the predominant cells for the first 24 hours, and are then followed by macrophages, which peak at 96 hours, and then by lymphocytes. It seems the polymorphonuclear leukocyte influx is a specific response to pleural injury and, either through itself or its interaction with the macrophage, plays a role in host defence mechanisms against the tubercle bacilli (12,13).
Clinical manifestations
Pleural is second only to lymphatic involvement as a site of extrapulmonary TB (7,14), and may occur in either primary or reactivation disease (15,16). In the USA 3-5% of TB patients are reported to have pleural disease (14), while the incidence of pleural involvement may be as high as 30% in high-burden TB settings (17,18). Where HIV is endemic, TB pleuritis is the most common cause of a lymphocytic effusion and thought to be caused by primary infection in 30% of patients (18). Most (HIV negative) patients with TB pleural effusions will present with an acute febrile illness characterised by a non-productive cough and pleuritic chest pain, but without an elevation in the peripheral white blood cell count (7,19). Night sweats, chills, weakness, dyspnoea, and weight loss are also frequently reported (20). TB pleural effusion may resolve spontaneously without treatment, but patients frequently develop active TB at a later date (21). In non-HIV endemic areas where reactivation is the predominant mechanism of TB disease, pleural involvement is reported to occur in 4% of cases. Such patients have a more insidious onset, are of older age and are more likely to be immunocompromised (18). Immunocompromised patients have been shown to have higher positive culture rates, thought to be due to impaired clearing of the organism (22).
Chronic TB empyema is less common, and represents a distinct entity of chronic, active infection within the pleural space. It is characterised by purulent fluid where virtually all the nucleated white blood cells are neutrophils, and can occur in several settings: (I) progression of a primary TB pleuritis; (II) direct extension of infection into the pleural space from thoracic lymph nodes or a subdiaphragmatic focus; (III) haematogenous spread; or (IV) following pneumonectomy (9). The majority of empyemas will resolve leaving a thickened, scarred, and calcified pleura (23). However, this process may be complicated by decompression through the chest wall (empyema necessitans) (24). Pneumothorax secondary to TB often heralds severe pulmonary involvement by the infectious process and the onset of bronchopleural fistula and empyema (23).
Pleural fibrosis or fibrothorax (Figure 1) is a well described complication of TB pleuritis (25). However, uncertainty remains to the exact prevalence, with reports varying between 5% and 55% (26,27). Further, pleural fibrosis may have long term clinical implications, with some studies reporting the association of residual pleural thickening (≥10 mm) with significant morbidity, including chronic chest pain, dyspnoea as well as impairment in lung function (25,28).
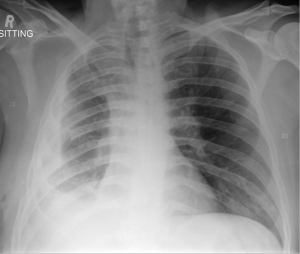
Chest X-ray
Pleural effusions secondary to TB are largely unilateral with a slight right-sided predominance, reported to occur in 55% of cases (Figure 2) (29). The effusions are typically small to moderate in size, occupying less than one-third of the hemithorax in approximately 80% of cases (29). However, neither the size nor side of the effusion has been reported to have bearing on prognosis (30).
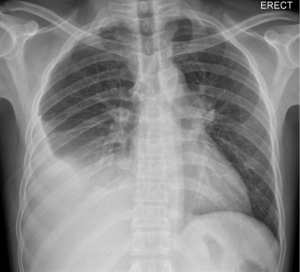
The coexistence of parenchymal disease in association with pleural effusion has been observed on chest radiograph in up to 50% of patients (7), and occurs on the same side in almost all cases (20). Observed parenchymal changes are located in the upper lobes in three quarters of cases, suggesting reactivation as the cause of TB. In the remaining patients, parenchymal disease is located in the lower lobe suggesting primary TB infection (20).
Ultrasonography
Thoracic ultrasound is now accepted as standard of care in performing thoracentesis and closed pleural biopsies (31). Detection of localised pleural thickening and other pleural abnormalities can direct the operator to a preferred biopsy site (32). However, apart from the performance of invasive diagnostic procedures, ultrasound may additionally assist in characterising the nature of the effusion (31). The ultrasonographic appearance of pleural effusions secondary to TB range from anechoic to complex septated or non-septated to even homogeneously echogenic effsuions (33,34).
Computed tomography (CT)
CT of the chest is currently the best imaging modality to visualise both pleura and lung parenchyma in TB pleural effusions (Figure 3). Apart from visualising the extent of the disease, CT can be used to assess TB empyema, which can be divided into three distinct phases. The exudative phase represents the initial uncomplicated effusion, followed by the fibrinopurulent phase, where CT typically shows thickened visceral and parietal pleurae separated by fluid, known as the “split pleura” sign. In the organizing phase, CT reveals a loculated pleural fluid collection with a thickened pleural peel and variable degree of calcification with or without proliferation of extrapleural fat (24).
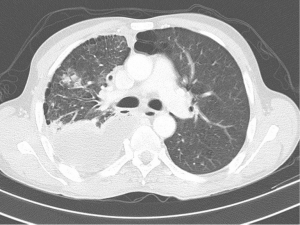
CT is more sensitive in diagnosing coexistence of parenchymal abnormalities when compared to conventional chest radiographs. In a recent study, more than 80% of patients had pulmonary parenchymal abnormalities when CT scanning was performed (35). The most common CT findings being micronodules in both the subpleural and peribronchovascular interstitium, with interlobular septal thickening, suggesting lymphatic spread of TB (35). Interestingly, in addition to these findings, a paradoxical response to treatment is not rare in patients with pleural TB, and is noted especially in young, previously healthy, male patients with subpleural nodules on initial CT scans (35).
Diagnosis
Principles
The gold standard for the diagnosis of TB pleuritis is detection of M. tuberculosis in the sputum, pleural fluid or pleural biopsy specimens, either by microscopy and/or culture, or the histological demonstration of caseating granulomas in the pleura along with acid fast bacilli (AFB) (18,28,34). In high burden settings, however, the diagnosis is frequently inferred in patients who present with a lymphocytic predominant exudate and high ADA level.
Sputum
It was previously believed that patients without overt coexisting parenchymal lesion are sputum negative and, therefore, noncontagious. Moreover, the mycobacterial culture rate in non-induced sputum is low. Reported sensitivities range from 0% to 30% (36). The method of sputum collection, however, is of crucial importance. Conde et al. (37) reported a yield of 52% on mycobacterial culture with induced single sputum specimens. Even in patients with normal underlying lung parenchyma on chest X-ray, the yield of sputum culture in induced samples approached 55%. Therefore, in patients with suspected TB pleural effusion it is important to obtain cultures on induced sputum samples, even in the absence of obvious parenchymal involvement. To our knowledge there are no studies evaluating the role of sputum nucleic acid amplification (NAA) molecular studies such as Xpert MTB/RIF in the context of TB pleural effusions. NAA tests may increase the diagnostic yield of sputum compared with conventional microscopy, but is unlikely to be more sensitive than culture.
Thoracentesis
Microscopy and culture
The macroscopic appearance is that of straw coloured fluid in more than 80% of cases (5). Microscopy for AFB in the pleural fluid can identify M. tuberculosis in fewer than 10% of cases. The exception to this is patients with HIV and tuberculous empyema, where yields may be higher (>20%) (9). Culture of the pleural fluid can be performed on either solid or liquid media as in the commercially available and widely used BACTEC MGIT semi-automated system (Becton-Dickinson, Franklin Lakes, NJ, USA), or with manual culture methods that might allow resistance testing at the same time such as the microscopic-observation drug susceptibility (MODS) assay (38). When using solid culture media, sensitivities reported have been low, in the range of 12% to 30%. However, it appears that liquid culture media display better sensitivities of up to 70%. A further benefit of using liquid media is the significantly shorter time required for culture results, being 2 weeks compared to the traditional 6 weeks for solid media (39).
Perhaps counter-intuitively, it does not appear that sending larger volumes of pleural fluid for culture improves diagnostic sensitivity. von Groote-Bidlingmaier et al. compared the yield of high and low pleural fluid volumes (100 vs. 5 mL) inoculated into liquid culture medium in patients with a high pre-test probability of TB (22). It was shown that the absolute yield was not significantly higher for the larger volume (53.5% vs. 50% respectively; P=0.75). In the same study, HIV-positive individuals had culture-positive pleural fluid more frequently than HIV-negative patients. This is presumably due to impaired bacterial clearance from the pleural space. Underlying immunosuppression as a contributor is corroborated by the known negative association of lymphocyte percentage in pleural fluid with the probability of positive culture of pleural fluid (5). The combination of pleural fluid and sputum cultures in the diagnostic workup of TB pleuritis seems a reasonable initial approach, with a combined diagnostic yield of almost 80% (5,22). In a recent study the reported diagnostic yield was 63% for effusion culture, 48% for sputum culture and 79% for the combination of effusion and sputum cultures, using liquid culture media (5).
Routine chemistry
The effusion is uniformly exudative having protein concentrations invariably >50, and >30 g/L in 50% to 77% of cases (40). The pleural fluid lactate dehydrogenase (LDH) level is elevated in approximately 75% of cases, with levels commonly exceeding 500 IU/L (7,20,40). The pleural fluid pH is usually less than 7.40 with values below 7.30 in about 20% of cases (20,40). A low pH and a low glucose concentration may be observed but are more characteristic of chronic tuberculous empyema than TB (41,42). In fact, a pleural fluid pH of <7.20 indicates a possible empyema, prompting the physician to consider drainage of the fluid. The pleural fluid glucose concentration in TB is normally between 3.3 and 5.6 mmol/L, with glucose levels <2.8 mmol/L seen in 7-20% of effusions, while extremely low glucose concentrations (<1.7 mmol/L) may occasionally be observed (20,40).
Adenosine deaminase (ADA)
ADA levels are most useful when there is a moderate to high suspicion of TB in patients with negative pleural fluid or biopsy cultures, and non-diagnostic histology (43). There is a wide range of cut-off values used by authors but in the majority of studies the most accurate threshold was found to range between 40 and 60 U/L (43). In a study of 254 patients with pleural TB, 99.6% had ADA more than 47 U/L (29) and in another group of 303 patients in a high TB prevalence population with exudative effusions, 58% had TB with a lymphocytic predominant effusion and ADA more than 50 U/L (44). The diagnostic usefulness of ADA depends not only on its sensitivity and specificity, but also on the local prevalence of TB. In populations with a high prevalence of TB and clinical suspicion of TB effusion, elevated ADA level might be considered as a confirmatory test justifying treatment initiation. In contrast, in countries with a low prevalence of TB, the negative predictive value remains high even though the positive predictive value of pleural ADA declines. This was illustrated by two studies done in populations with low TB prevalence and non-TB lymphocytic effusions, in which 97% and 98% had ADA levels less than 40 U/L (45,46). Therefore, a negative ADA test may justify abandoning further diagnostic procedures for TB, and pursuing alternative diagnoses.
When interpreting ADA levels, the clinician must additionally be aware of situations which may increase the likelihood of both the false-negative and false-positive ADA results. In the early phase of the disease low levels of ADA in the pleural fluid may be found, giving rise to a false negative result. However, ADA levels will invariably be elevated if thoracentesis is repeated a few days later (47). Additional care should also be taken when interpreting pleural ADA levels in elderly patients and/or current smokers, as ADA levels may be low in such TB patients (48). Conversely, raised ADA levels may be observed in a number of conditions potentially leading to a false positive diagnosis of TB. These include rheumatoid effusion, empyema due to other bacteria, mesothelioma, lung cancer, parapneumonic effusion, and haematological malignancies (47,48).
The diagnostic accuracy of ADA can be improved by measuring different ADA isoenzymes. ADA-2 is increased in TB effusions, while ADA-1 is increased in other bacterial empyemas (49), and distinguishing between these two principal isoenzymes can increase the specificity of ADA for diagnosing TB. Use of the ADA-2 isoenzyme measurement increased the specificity for TB from 91% to 96% (50) and 92.1% to 98.6% (51), in two different studies.
Although it has been suggested that ADA might be a less sensitive marker of TB in immunocompromised patients, there is currently little evidence to support this view. Baba et al. (52) demonstrated that ADA is a reliable marker of pleural TB in HIV-positive patients, even for those with low CD4 counts, while Chung et al. (53) confirmed that ADA is an accurate marker in renal transplant recipients.
Nucleated cell count and cytology
The cell count performed usually reveals a nucleated (white) cell count between 1,000 and 6,000 cells/mm3 (29), and has a T lymphocyte predominance in 60% to 90% of cases (7,20,40). However, the predominant nucleated cell type can vary depending on timing of collection of the pleural fluid. Fluid collected in the first few days may exhibit a neutrophil predominant effusion, while lymphocytes tend to dominate thereafter (12,13). A lymphocytic predominant effusion may be defined as one with more than 75% lymphocytes and/or a lymphocyte to neutrophil ratio more than 0.75 (18). When a lymphocyte neutrophil ratio of 0.75 or greater is used in combination with ADA, the sensitivity, specificity, positive predictive value, negative predictive value, and efficiency for the identification of TB were reported at 88%, 95%, 95%, 88%, and 92%, respectively (44). Other cell types are much less common in TB pleural effusions, with eosinophils being rare unless the patient has had a pneumothorax or haemothorax near the time of pleural analysis (54). The presence of more than 5% mesothelial cells is unusual (55). Cytological examination of the pleural fluid is routinely done, as previously stated, as a malignant pleural effusion (MPE) may present with a lymphocytic predominant exudate as well as a high ADA.
Additional pleural fluid assays and biomarkers
IFN-γ is an important Th1 cytokine and is important in the host’s immune response to mycobacterial infection. Measurement of unstimulated pleural fluid IFN-γ concentration by commercially available enzyme-linked immunosorbent assay kits is considered a useful diagnostic tool to diagnose TB pleuritis (49,56). However, concerns similar to that for ADA around the specificity of the test have been expressed. In one study including 145 patients with TB, an IFN-γ concentration >140 pg/mL had sensitivity and specificity of 94% and 92%, respectively (29), while another study (n=66) of exudative, lymphocytic pleural effusions using a cut-off value of 240 pg/mL, found a sensitivity and specificity of 95% and 96% (57). A meta-analysis which included 22 studies to estimate the diagnostic accuracy of IFN-γ for pleural TB, noted sensitivity and specificity of 89% and 97%, respectively (58), while other series have described lower sensitivities (59). More recently, IFN-γ was compared to ADA in a TB-endemic setting and showed a significantly greater sensitivity than ADA (IFN-γcut-off: 107.7 pg/mL) (60). Thus, it appears that in a high burden TB setting, IFN-γ may have a similar potential utility to the current use of ADA. However, the test is not yet widely available in clinical practice and important information is not yet available regarding false positives and negatives situations, as well as its utility in low TB burden settings, thus preventing definitive recommendations for its use.
NAA techniques for evaluation of TB pleuritis in HIV-negative patients appear to have high specificity but relatively low sensitivity. In a meta-analysis of HIV-negative patients, NAA tests had relatively low sensitivity (62%) but high specificity (98%) for diagnosing TB (61). The relatively high sensitivity of the Xpert MTB/RIF NAA assay contrasts with the sensitivity and specificity of 25% and 100%, respectively, reported in a series of 20 cases of confirmed TB from a region with high TB prevalence (62). More recently the Xpert assay was tested in a high prevalence HIV/TB setting and the sensitivity and specificity of Xpert MTB/RIF test were 28.7% and 96.6%, respectively, while the respective positive and negative predictive values were 96.1% and 31.1% (63). Xpert MTB/RIF test on pleural fluid does not accurately diagnose pleural TB and therefore cannot be used as an initial evaluation test in patients with suspected pleural TB.
Lysozyme is an enzyme found in the cytoplasmic granules of neutrophils which hydrolyses bacterial cell walls. Pleural fluid lysozyme concentrations are >15 mg/dL in 80% of cases of pleural TB (64,65), with highest concentrations found in empyemas due to both tuberculous and non-tuberculous bacteria (65). However, lysozyme is not specific for infection and concentrations may also be elevated in MPE (64). The pleural fluid: serum lysozyme ratio may be more useful than the absolute value of lysozyme. A ratio >1.2 has been reported to diagnose empyema with a 100% sensitivity for TB pleuritis and 95% specificity (65). However, in a separate study of 276 pleural effusions of heterogeneous aetiology, a threshold ratio of 1.1 was associated with a sensitivity of only 67% with specificity of 90% (64), thus casting doubt on its utility.
Additional biomarkers used in differentiating MPE from TB pleuritis are simultaneous measurements of pleural DcR3 and TNF-sR1 (66), while a further study assessed the specificity and accuracy of combinations of TNF-α and ADA2 (67). Other novel biomarkers that may in the future, aid diagnosis of TB pleuritis include: hyaluronic acid, neopterin, leptin and fibronectin concentration in pleural fluid, although there is currently limited data, with variable sensitivities and specificities being reported (68). Urinary lipoarabinomannan ELISA has recently been evaluated for the diagnosis of TB in HIV-infected patients with paucibacillary disease and may also be useful in the diagnosis of pleural TB (69).
Pleural biopsy
The presence of caseating granulomas containing acid-fast bacilli on histological examination of the pleural surface is diagnostic of TB pleuritis (9). The demonstration of acid-fast bacilli is not an absolute requirement; the presence of caseating granulomas in high burden settings is considered adequate (18). Pleural tissue can be harvested either by means of closed pleural biopsies, thoracoscopy or open surgical biopsies (34). Access to thoracoscopy and open surgical biopsies is limited in many parts of the world where TB is endemic and, therefore, if pleural fluid analysis proves inconclusive, closed biopsy is the preferred next investigation. Ultrasound-guided biopsy techniques have gained popularity amongst pulmonary physicians and are becoming the standard of care for obtaining pleural tissue in patients with suspected TB pleuritis. Medical thoracoscopy is reserved for the small number of cases where closed pleural biopsies fail to provide a diagnosis (18,34). Ultrasound-guided pleural biopsy has a diagnostic yield of up to 90% for pleural TB, which is not surprising given the diffuse nature of the disease (22).
Some centres prefer to use the Tru-Cut needle biopsies over the more conventional Abrams needle. Current evidence, however, still favours the Abrams needle as demonstrated by one study which found an overall diagnostic yield of 81.8% for the Abrams needle compared to 65% for the Tru-cut needle (35).
Because of sampling error, sensitivity generally increases with the number of biopsies taken, and biopsies taken during medical thoracoscopy have previously been found to have a diagnostic sensitivity of 100% (34). With medical thoracoscopy direct visualisation of the diseased pleura is possible, using either a rigid or a flexible endoscope, which guides the operator to the most appropriate biopsy site. Due to the reported high yield and less invasive nature of thoracoscopy, open surgical biopsies are seldom required.
Practical diagnostic approach
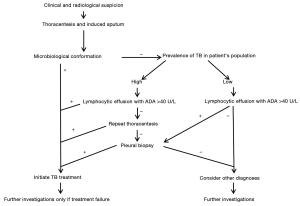
A practical suggested diagnostic approach, based on pre-test probability, is presented in Figure 4. If the nucleated cell count is lymphocytic predominant with a high ADA (>40 U/L), and the background TB prevalence is high, it is prudent to initiate TB therapy. However, in a low prevalence population further confirmation is required by obtaining pleural biopsies for both histology and culture. Thoracoscopy has a superior diagnostic yield for both pleural malignancy and TB, and is therefore considered by many to be the investigation of choice in exudative pleural effusions where a thoracentesis was non-diagnostic. Pleural fluid findings are known to evolve from early neutrophil to lymphocytic predominance in TB effusions, and the value of repeat thoracentesis has been shown to significantly increase the diagnostic yield. In fact, we have shown that 77.8% of cases of confirmed pleural TB could be diagnosed on a second pleural aspiration in patients who previously had at least one non-diagnostic thoracentesis. In a high burden and resource constrained setting it may therefore be appropriate to repeat ultrasound guided thoracentesis before proceeding to pleural biopsy (70). An image-assisted second thoracentesis combined with an image-assisted pleural biopsy with either an Abrams needle or cutting needle (depending on the clinical setting and imagery) may be an acceptable alternative to thoracoscopy, particularly when the probability of TB pleural effusion is high.
Treatment
The medical treatment for TB pleural effusion is the same as for pulmonary TB, and is consistent with the theory that the majority of pleural TB cases develop from pulmonary disease. The expected resolution of TB pleural effusion is variable, and assuming appropriate therapy, fever usually resolves within 2 weeks with reabsorption of the pleural fluid within 6 weeks. Naturally this will depend on the burden of disease in the individual, and size of the effusion and resorption may take up to 2-4 months.
The current evidence on the role of surgical intervention is limited and difficult to interpret, and intercostal drainage is traditionally not offered to patients unless severe dyspnoea is present (71,72). A recent study by Bhuniya et al. (73) investigated the use of early pleural drainage (using pleural manometry) in addition to standard anti-TB therapy, compared to standard therapy alone; and demonstrated significant differences after 6 months in lung function. The drainage group had a forced expiratory volume in the first second (FEV1) of 87.6% as compared to the control group of 84.9% (P=0.02), with forced vital capacity (FVC) of 84.5% and 83.3% (P<0.01), respectively. The long term clinical relevance of these small differences in lung function is not known. However, these authors did report a lower incidence of residual pleural thickening in drained patients and also commented that patients with therapeutic thoracentesis experienced immediate relief from dyspnoea after drainage. Earlier studies reported that residual pleural thickening ≥10 mm can cause significant clinical symptoms in patients with TB pleural effusion, with reported incidences varying from 26% to 50.4% (25,26,73,74). In unpublished data, we found that patients with confirmed TB pleural effusions, randomised to therapeutic pleural drainage, showed significantly superior improvements in several lung function parameters after 3 and 6 months follow-up, despite complete drainage being achieved in less than half of all patients.
In selected patients, administration of corticosteroids can shorten the duration of fever and time to fluid resorption, although the risks and benefits of corticosteroids in this setting have not been well defined (75,76). Currently data is insufficient to support routine adjunctive use of corticosteroids for TB pleuritis (77).
Conclusions
On a global scale, TB remains one of the most frequent causes of pleural effusions. Our understanding of the pathogenesis of the disease has evolved and what was once thought to be an effusion as a result of a pure delayed hypersensitivity reaction is now believed to be the consequence of direct infection of the pleural space with a resultant lymphocyte driven immunological response. Pulmonary involvement is more common than previously believed and induced sputum, which is grossly underutilised, can be diagnostic in approximately 50%. The gold standard for the diagnosis of tuberculous pleuritis remains the detection of M. tuberculosis in pleural fluid or pleural biopsy specimens, either by microscopy and/or culture, or the histological demonstration of caseating granulomas in the pleura along with AFB. In high burden settings, however, the diagnosis is frequently inferred in patients who presents with a lymphocytic predominant exudate with a high ADA level, which is a valuable adjunct in the diagnostic evaluation. ADA is generally readily accessible, and together with lymphocyte predominance justifies treatment initiation in patients with a high pre-test probability. However, false-negative and false-positive results remain problematic. When adding closed pleural biopsy to ADA and lymphocyte count, diagnostic accuracy approaches that of thoracoscopy. The role of other biomarkers is less well described. Unstimulated pleural fluid IFN-γ or other newer assays, might lead to greater diagnostic accuracy, but further studies are required. Early pleural drainage may have a role in selected cases, but more research is required to validate its use.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev 2003;16:463-96. [PubMed]
- World Health Organization. Global tuberculosis report 2014. Geneva: World Health Organization, 2014. Available online: http://www.who.int/tb/publications/global_report/en/. Accessed 10 December 2014.
- Porcel JM. Tuberculous pleural effusion. Lung 2009;187:263-70. [PubMed]
- Leibowitz S, Kennedy L, Lessof MH. The tuberculin reaction in the pleural cavity and its suppression by antilymphocyte serum. Br J Exp Pathol 1973;54:152-62. [PubMed]
- Ruan SY, Chuang YC, Wang JY, et al. Revisiting tuberculous pleurisy: pleural fluid characteristics and diagnostic yield of mycobacterial culture in an endemic area. Thorax 2012;67:822-7. [PubMed]
- Koch R. Die Aetiologie der Tuberculose. Berliner Klinische Wochenschrift 1882;19:221-30.
- Seibert AF, Haynes J Jr, Middleton R, et al. Tuberculous pleural effusion. Twenty-year experience. Chest 1991;99:883-6. [PubMed]
- Sharma SK, Mitra DK, Balamurugan A, et al. Cytokine polarization in miliary and pleural tuberculosis. J Clin Immunol 2002;22:345-52. [PubMed]
- Gopi A, Madhavan SM, Sharma SK, et al. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest 2007;131:880-9. [PubMed]
- Rossi GA, Balbi B, Manca F. Tuberculous pleural effusions. Evidence for selective presence of PPD-specific T-lymphocytes at site of inflammation in the early phase of the infection. Am Rev Respir Dis 1987;136:575-9. [PubMed]
- Mitra DK, Sharma SK, Dinda AK, et al. Polarized helper T cells in tubercular pleural effusion: phenotypic identity and selective recruitment. Eur J Immunol 2005;35:2367-75. [PubMed]
- Antony VB, Repine JE, Harada RN, et al. Inflammatory responses in experimental tuberculosis pleurisy. Acta Cytol 1983;27:355-61. [PubMed]
- Antony VB, Sahn SA, Antony AC, et al. Bacillus Calmette-Guérin-stimulated neutrophils release chemotaxins for monocytes in rabbit pleural spaces and in vitro. J Clin Invest 1985;76:1514-21. [PubMed]
- Baumann MH, Nolan R, Petrini M, et al. Pleural tuberculosis in the United States: incidence and drug resistance. Chest 2007;131:1125-32. [PubMed]
- Kim HJ, Lee HJ, Kwon SY, et al. The prevalence of pulmonary parenchymal tuberculosis in patients with tuberculous pleuritis. Chest 2006;129:1253-8. [PubMed]
- Torgersen J, Dorman SE, Baruch N, et al. Molecular epidemiology of pleural and other extrapulmonary tuberculosis: a Maryland state review. Clin Infect Dis 2006;42:1375-82. [PubMed]
- Saks AM, Posner R. Tuberculosis in HIV positive patients in South Africa: a comparative radiological study with HIV negative patients. Clin Radiol 1992;46:387-90. [PubMed]
- Diacon AH, Van de Wal BW, Wyser C, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J 2003;22:589-91. [PubMed]
- Onyenekwu CP, Zemlin AE, Erasmus RT. High pleural fluid adenosine deaminase levels: a valuable tool for rapid diagnosis of pleural TB in a middle-income country with a high TB/HIV burden. S Afr Med J 2014;104:200-3. [PubMed]
- Berger HW, Mejia E. Tuberculous pleurisy. Chest 1973;63:88-92. [PubMed]
- Roper WH, Waring JJ. Primary serofibrinous pleural effusion in military personnel. Am Rev Tuberc 1955;71:616-34. [PubMed]
- von Groote-Bidlingmaier F, Koegelenberg CF, Bolliger CT, et al. The yield of different pleural fluid volumes for Mycobacterium tuberculosis culture. Thorax 2013;68:290-1. [PubMed]
- Sonmezoglu Y, Turna A, Cevik A, et al. Factors affecting morbidity in chronic tuberculous empyema. Thorac Cardiovasc Surg 2008;56:99-102. [PubMed]
- Hwang SH, Mokpo K. Pictorial Review of Tuberculosis involving the Pleura. The European Society of Radiology Conference, 2011.
- Bolliger CT, de Kock MA. Influence of a fibrothorax on the flow/volume curve. Respiration 1988;54:197-200. [PubMed]
- Candela A, Andujar J, Hernández L, et al. Functional sequelae of tuberculous pleurisy in patients correctly treated. Chest 2003;123:1996-2000. [PubMed]
- Barbas CS, Cukier A, de Varvalho CR, et al. The relationship between pleural fluid findings and the development of pleural thickening in patients with pleural tuberculosis. Chest 1991;100:1264-7. [PubMed]
- Light RW. Update on tuberculous pleural effusion. Respirology 2010;15:451-8. [PubMed]
- Valdés L, Alvarez D, San José E, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med 1998;158:2017-21. [PubMed]
- Sibley JC. A study of 200 cases of tuberculous pleurisy with effusion. Am Rev Tuberc 1950;62:314-23. [PubMed]
- Koegelenberg CF, von Groote-Bidlingmaier F, Bolliger CT. Transthoracic ultrasonography for the respiratory physician. Respiration 2012;84:337-50. [PubMed]
- Koegelenberg CF, Diacon AH. Image-guided pleural biopsy. Curr Opin Pulm Med 2013;19:368-73. [PubMed]
- Koegelenberg CF, Bolliger CT, Irusen EM, et al. The diagnostic yield and safety of ultrasound-assisted transthoracic fine-needle aspiration of drowned lung. Respiration 2011;81:26-31. [PubMed]
- Koegelenberg CF, Bolliger CT, Theron J, et al. Direct comparison of the diagnostic yield of ultrasound-assisted Abrams and Tru-Cut needle biopsies for pleural tuberculosis. Thorax 2010;65:857-62. [PubMed]
- Ko JM, Park HJ, Kim CH. Pulmonary changes of pleural TB: up-to-date CT imaging. Chest 2014;146:1604-11. [PubMed]
- Udwadia ZF, Sen T. Pleural tuberculosis: an update. Curr Opin Pulm Med 2010;16:399-406. [PubMed]
- Conde MB, Loivos AC, Rezende VM, et al. Yield of sputum induction in the diagnosis of pleural tuberculosis. Am J Respir Crit Care Med 2003;167:723-5. [PubMed]
- Tovar M, Siedner MJ, Gilman RH, et al. Improved diagnosis of pleural tuberculosis using the microscopic- observation drug-susceptibility technique. Clin Infect Dis 2008;46:909-12. [PubMed]
- Maartens G, Bateman ED. Tuberculous pleural effusions: increased culture yield with bedside inoculation of pleural fluid and poor diagnostic value of adenosine deaminase. Thorax 1991;46:96-9. [PubMed]
- Epstein DM, Kline LR, Albelda SM, et al. Tuberculous pleural effusions. Chest 1987;91:106-9. [PubMed]
- Ferrer J. Tuberculous pleural effusion and tuberculous empyema. Semin Respir Crit Care Med 2001;22:637-46. [PubMed]
- Malhotra P, Aggarwal AN, Agarwal R, et al. Clinical characteristics and outcomes of empyema thoracis in 117 patients: a comparative analysis of tuberculous vs. non-tuberculous aetiologies. Respir Med 2007;101:423-30. [PubMed]
- Krenke R, Korczyński P. Use of pleural fluid levels of adenosine deaminase and interferon gamma in the diagnosis of tuberculous pleuritis. Curr Opin Pulm Med 2010;16:367-75. [PubMed]
- Burgess LJ, Maritz FJ, Le Roux I, et al. Combined use of pleural adenosine deaminase with lymphocyte/neutrophil ratio. Increased specificity for the diagnosis of tuberculous pleuritis. Chest 1996;109:414-9. [PubMed]
- Lee YC, Rogers JT, Rodriguez RM, et al. Adenosine deaminase levels in nontuberculous lymphocytic pleural effusions. Chest 2001;120:356-61. [PubMed]
- Jiménez Castro D, Díaz Nuevo G, Pérez-Rodríguez E, et al. Diagnostic value of adenosine deaminase in nontuberculous lymphocytic pleural effusions. Eur Respir J 2003;21:220-4. [PubMed]
- Valdés L, Pose A, San José E, et al. Tuberculous pleural effusions. Eur J Intern Med 2003;14:77-88. [PubMed]
- Lee SJ, Kim HS, Lee SH, et al. Factors influencing pleural adenosine deaminase level in patients with tuberculous pleurisy. Am J Med Sci 2014;348:362-5. [PubMed]
- Yurt S, Küçükergin C, Yigitbas BA, et al. Diagnostic utility of serum and pleural levels of adenosine deaminase 1-2, and interferon-γ in the diagnosis of pleural tuberculosis. Multidiscip Respir Med 2014;9:12. [PubMed]
- Valdés L, San José E, Alvarez D, et al. Adenosine deaminase (ADA) isoenzyme analysis in pleural effusions: diagnostic role, and relevance to the origin of increased ADA in tuberculous pleurisy. Eur Respir J 1996;9:747-51. [PubMed]
- Pérez-Rodríguez E, Pérez Walton IJ, Sanchez Hernández JJ, et al. ADA1/ADAp ratio in pleural tuberculosis: an excellent diagnostic parameter in pleural fluid. Respir Med 1999;93:816-21. [PubMed]
- Baba K, Hoosen AA, Langeland N, et al. Adenosine deaminase activity is a sensitive marker for the diagnosis of tuberculous pleuritis in patients with very low CD4 counts. PLoS One 2008;3:e2788. [PubMed]
- Chung JH, Kim YS, Kim SI, et al. The diagnostic value of the adenosine deaminase activity in the pleural fluid of renal transplant patients with tuberculous pleural effusion. Yonsei Med J 2004;45:661-4. [PubMed]
- Light RW. Tuberculous Pleural Effusions. In: Light RW. editor. Pleural Diseases. 5th Edition. Baltimore: Lippincott, Williams and Wilkins, 2007:211-24.
- Koegelenberg CF, Diacon AH. Diagnosis of TB Pleural Effusions. In: Light RW, Lee YC. editors. International Pleural Newsletter. Oxford, UK: Emma Hedley, 2007:6-7.
- Aoe K, Hiraki A, Murakami T, et al. Diagnostic significance of interferon-gamma in tuberculous pleural effusions. Chest 2003;123:740-4. [PubMed]
- Wongtim S, Silachamroon U, Ruxrungtham K, et al. Interferon gamma for diagnosing tuberculous pleural effusions. Thorax 1999;54:921-4. [PubMed]
- Jiang J, Shi HZ, Liang QL, et al. Diagnostic value of interferon-gamma in tuberculous pleurisy: a metaanalysis. Chest 2007;131:1133-41. [PubMed]
- Villegas MV, Labrada LA, Saravia NG. Evaluation of polymerase chain reaction, adenosine deaminase, and interferon-gamma in pleural fluid for the differential diagnosis of pleural tuberculosis. Chest 2000;118:1355-64. [PubMed]
- Meldau R, Peter J, Theron G, et al. Comparison of same day diagnostic tools including Gene Xpert and unstimulated IFN-γ for the evaluation of pleural tuberculosis: a prospective cohort study. BMC Pulm Med 2014;14:58. [PubMed]
- Pai M, Flores LL, Hubbard A, et al. Nucleic acid amplification tests in the diagnosis of tuberculous pleuritis: a systematic review and meta-analysis. BMC Infect Dis 2004;4:6. [PubMed]
- Friedrich SO, von Groote-Bidlingmaier F, Diacon AH. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. J Clin Microbiol 2011;49:4341-2. [PubMed]
- Lusiba JK, Nakiyingi L, Kirenga BJ, et al. Evaluation of Cepheid’s Xpert MTB/Rif test on pleural fluid in the diagnosis of pleural tuberculosis in a high prevalence HIV/TB setting. PLoS One 2014;9:e102702. [PubMed]
- Valdés L, San José E, Alvarez D, et al. Diagnosis of tuberculous pleurisy using the biologic parameters adenosine deaminase, lysozyme, and interferon gamma. Chest 1993;103:458-65. [PubMed]
- Verea Hernando HR, Masa Jimenez JF, Dominguez Juncal L, et al. Meaning and diagnostic value of determining the lysozyme level of pleural fluid. Chest 1987;91:342-5. [PubMed]
- Shu CC, Wang JY, Hsu CL, et al. Diagnostic role of inflammatory and anti-inflammatory cytokines and effector molecules of cytotoxic T lymphocytes in tuberculous pleural effusion. Respirology 2015;20:147-54. [PubMed]
- Li M, Wang H, Wang X, et al. Diagnostic accuracy of tumor necrosis factor-alpha, interferon-gamma, interlukine-10 and adenosine deaminase 2 in differential diagnosis between tuberculous pleural effusion and malignant pleural effusion. J Cardiothorac Surg 2014;9:118. [PubMed]
- Trajman A, Pai M, Dheda K, et al. Novel tests for diagnosing tuberculous pleural effusion: what works and what does not? Eur Respir J 2008;31:1098-106. [PubMed]
- Peter JG, Theron G, Dheda K. Can point-of-care urine LAM strip testing for tuberculosis add value to clinical decision making in hospitalised HIV-infected persons? PLoS One 2013;8:e54875. [PubMed]
- Koegelenberg CF, von Groote-Bidlingmaier F, Bruwer JW, et al. An image-guided diagnostic pathway for undiagnosed pleural exudates. Am J Respir Crit Care Med 2014;189:A5478.
- Bagheri R, Haghi SZ, Rajabi MT, et al. Outcomes following surgery for complicated tuberculosis: analysis of 108 patients. Thorac Cardiovasc Surg 2013;61:154-8. [PubMed]
- Byun CS, Chung KY, Narm KS, et al. Early and Long-term Outcomes of Pneumonectomy for Treating Sequelae of Pulmonary Tuberculosis. Korean J Thorac Cardiovasc Surg 2012;45:110-5. [PubMed]
- Bhuniya S, Arunabha DC, Choudhury S, et al. Role of therapeutic thoracentesis in tuberculous pleural effusion. Ann Thorac Med 2012;7:215-9. [PubMed]
- Lai YF, Chao TY, Wang YH, et al. Pigtail drainage in the treatment of tuberculous pleural effusions: a randomised study. Thorax 2003;58:149-51. [PubMed]
- Lee CH, Wang WJ, Lan RS, et al. Corticosteroids in the treatment of tuberculous pleurisy. A double-blind, placebo-controlled, randomized study. Chest 1988;94:1256-9. [PubMed]
- Matchaba PT, Volmink J. Steroids for treating tuberculous pleurisy. Cochrane Database Syst Rev 2000;CD001876. [PubMed]
- Engel ME, Matchaba PT, Volmink J. Corticosteroids for tuberculous pleurisy. Cochrane Database Syst Rev 2007;CD001876. [PubMed]


