
Evolution of chest CT manifestations of COVID-19: a longitudinal study
Introduction
As of March 11,2020, the coronavirus disease 2019 (COVID-19) pneumonia, which initially emerged in Wuhan city, China, has led to 118,000 infections and 4,291 fatalities in 114 countries (1-4). It poses a great threaten to the global public health and human life.
In confronting the tremendous threat of COVID-19, many important scientific issues need to be resolved immediately. Among these, imaging examinations (chest X-ray and CT) are important methods for diagnosing COVID-19. However, current imaging studies on COVID-19 are mainly cross-sectional studies (5-11). Systematic imaging studies regarding the evolving patterns of COVID-19 are still insufficient. Therefore, it is necessary to conduct studies on the image evolution of COVID-19 to explore its evolving patterns from the perspective of radiology.
In this study, we aim to investigate the evolution of chest CT features and outcome patterns of COVID-19 by analyzing the imaging and clinical data of 22 COVID-19 patients treated in our hospital. Such analysis will provide a basis for clinical diagnosis and treatment. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1363).
Methods
Study design and participants
This was a retrospective, single-center, observational study. A total of 76 patients with COVID-19 confirmed by RT-PCR tests of sputum, throat swabs, and lower respiratory tract secretion specimens at Taizhou Enze Hospital from January 17, 2020 to February 15, 2020 were selected. The inclusion criteria were: (I) the patient’s first visit was to our hospital, and subsequent clinical treatments and examinations were all conducted at our institution; (II) the clinical treatment plan and laboratory examination data were complete; (III) the patient received at least two chest CT examinations. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of Taizhou Enze Hospital. Because of the retrospective nature of the study, patient consent for inclusion was waived.
Clinical information collection
The collected data included epidemiological history (history of Wuhan residence or exposure), clinical data (demographic data, clinical symptoms, physical signs, laboratory test indicators, complications, and clinical outcomes) and image data (dynamic evolving characteristics of chest CT).
Statistical analysis
Continuous data conforming to the normal distribution were expressed as the mean ± standard deviation (mean ± SD), otherwise expressed using the median; categorical variables were represented by frequency. All statistical analyses were performed using SPSS 26.0 software.
Results
General clinical information
A total of 22 COVID-19 patients (13 males and 9 females) were included in this study, all of whom were diagnosed with mild or ordinary type COVID-19 (12). Age of onset was 20 to 74 years old, with a median age of 45 years. All patients had a history of Wuhan residence or exposure in Wuhan. The median time from onset of the disease to admission was 1 day (0–7 days), and the median time from onset to diagnosis was 3 days (0–9 days). Among the patients, 19 patients had a fever at the time of onset; 8 patients had a cough, of whom 6 had a dry cough and 2 had sputum; other symptoms included diarrhea (n=3), pharyngalgia (n=2), runny nose (n=1), fatigue (n=1), and dyspnea (n=1) (Table 1).
Full table
Laboratory information
Eleven patients had lymphopenia, 4 patients had thrombopenia, and 3 patients had leukopenia and neutropenia. Besides, 8 patients had hypoalbuminemia, and 8 patients had myoglobinemia. In addition, 12 patients had CRP values higher than normal, and 7 patients had an ESR higher than normal (Table 2).
Full table
Imaging results
General information on imaging examination
All 22 COVID-19 patients underwent two chest CT examinations during hospitalization, 20 patients underwent three chest CT examinations, and 11 patients underwent four chest CT examinations. The median interval time was 3 days (2–10 days) between the first and second CT examinations, 5 days (3–18 days) between the second and third CT examinations, and 9 days (4–16 days) between the third and fourth CT examinations (http://cdn.amegroups.cn/static/application/f60ac1df948fd97212228bd6cf343dbe.docx).
Imaging manifestations of the patient’s first CT examination
The lesions of 22 patients were located in the subpleural regions of the bilateral lungs, and those of 15 patients were located in the middle and lower lobes of the bilateral lungs. The first CT revealed the following signs: 18 patients showed single or multiple patchy or nodular ground-glass opacity (GGO) lesions, and 7 cases showed lung consolidation. Other accompanying signs included 5 cases of interlobular septal thickening inside the GGO lesions, 4 cases of fibrosis-like stripes, 2 cases of ground-glass nodules (GGNs) with reversed halo sign, 2 cases of air bronchogram sign, and 2 cases of vascular thickening. One patient showed no abnormalities in imaging (http://cdn.amegroups.cn/static/application/f60ac1df948fd97212228bd6cf343dbe.docx).
Evolution of main CT image manifestations
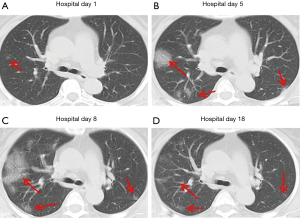
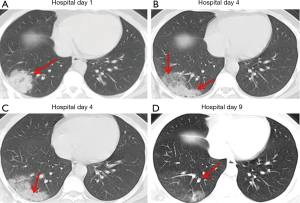
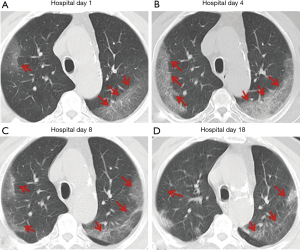
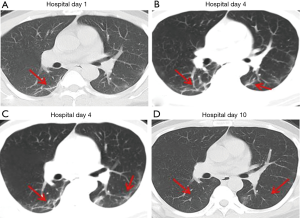
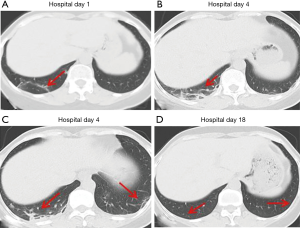
Over time, GGO in the lungs initially increased in number and density, or even progressed to consolidation, and later these GGO lesions gradually decreased in number and density or disappeared (Table 3, Figures 1,2,3). Besides, the lung consolidation demonstrated a trend of first increasing in number and then decreasing in number or disappearing over an extended time (Table 3, Figure 4). In addition, the signs of interlobular septal thickening within the GGO lesions demonstrated a trend of an increased size and density, and even the crazy-paving sign, followed by a decrease in size and reduced or disappeared density (Table 3, Figure 5). The fibrosis-like stripes also showed a trend of increasing in number first, and then decreasing in number or disappearing (Table 3, Figures 6,7,8,9).

Full table
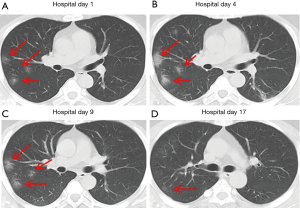
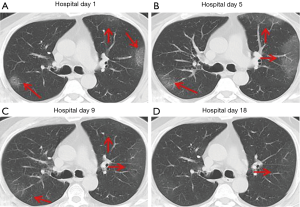
Imaging staging
Based on the dynamic evolution and outcome characteristics of the CT images of this group of cases, this study divided the dynamic changes of COIVD-19 into four stages: early stage (lung lesions start to appear), progressing stage (lung lesions clearly increase in number or new lesions appear), recovery stage (lung lesions begin to decrease in number) and dissipation stage (lung lesions clearly decrease in number or disappear).
Evolution of time points of imaging staging
The hospitalization time points and the disease time points at the time of CT examinations of this group of patients were classified according to imaging stages, and the disease time points of each imaging stage in the course of disease evolution were obtained (Table S1). In this study, the median time for the appearance of early stage imaging manifestations was 3 days (1–8 days) after onset, the median time of the progressing stage was 7 days (4–17 days) after onset, the median time of the recovery stage was 10 days (8–14 days) after onset, and the median time of the dissipation period was 19.5 days (11–25 days) after onset.
Correlations between clinical and radiological findings
In the present study, the correlation between patients’ clinical status (fever and cough) and the CT results was observed. However, the correlations between laboratory data and the CT results were not observed. For 19 patients with fever as the initial symptom, the median duration of fever was 4 days (2–19 days). Fourteen patients experienced the disappearance of their fever during the progressing stage (stage II), while 4 patients experienced such disappearance during the recovery stage (stage III), and 1 patient became fever free during the dissipation stage (stage IV). For 6 patients with a cough as the initial symptom, the median duration of the cough was 13.5 days (10–20 days). All 6 patients experienced the disappearance of the cough during the dissipation stage (stage IV).
Prognosis
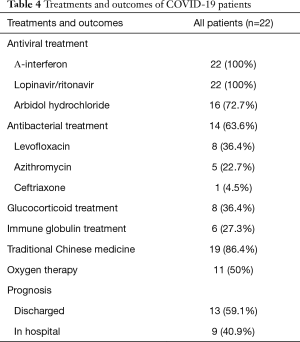
After active treatment with oxygen therapy, antiviral and antibacterial drugs, and glucocorticoids, the patients in this study generally showed a good prognosis. In the present study, methylprednisolone sodium succinate (40 mg/qd) was used through an intravenous drip for three days. As of February 15, 2020, 13 patients had been discharged, 9 patients were still hospitalized, and no serious complications or deaths occurred (Table 4).
Full table
Discussion
COVID-19 is extremely contagious and spreads rapidly, making the population generally susceptible and posing a significant threat to public health security. Early diagnosis and isolated treatment are important ways of controlling the epidemic. However, it is difficult to make a clinical diagnosis of COVID-19 because some infected patients lack a clear history of epidemiological exposure and clinical features as well as specific laboratory indicators. Imaging examinations (chest X-ray and CT) are important means of early diagnosis. However, currently, systematic imaging studies on COVID-19 are rare, and most of them are cross-sectional studies. Thus, the imaging evolution of COVID-19 remains unclear. Therefore, this study focused on the chest CT manifestations of COVID-19 and its CT evolving process to explore its inherent outcomes.
We found that common CT manifestations in the early stage of COVID-19 were single or multiple localized GGOs or nodules (Figure 1A), single or multiple small patchy GGOs (Figure 2A), or large GGOs (Figure 5). Most GGOs had unclear edges, whereas some had clear edges. The lesions were mostly distributed in the middle and lower lobes, and were located mostly in the subpleural regions of the bilateral lungs (Figures 1-11). In addition, other common CT manifestation was single or multiple lung consolidation (Figure 4A). Some GGO lesions were accompanied by interlobular septal thickening within the lesions and an increase of the small blood vessel network, which is similar to fine grid-like shadows (Figure 5A) or a crazy-paving sign (Figure 5B). Other accompanying signs included fibrosis-like stripes (Figures 7A,8A,9C), GGNs with a reversed halo sign (Figure 10A,11A), air bronchogram sign (Figure 4A), and vascular thickening inside the GGO lesions. The above lesions could exist alone or coexist with other lesions. Over time, GGO in the lungs initially increased in number and density and even became consolidation. Later, these lesions gradually decreased or disappeared. The lung consolidation demonstrated a trend of first increasing in number and then decreasing in number or disappearing. Based on the evolution of these major CT signs, the dynamic imaging changes of COVID-19 can be divided into four stages: early stage (stage I), progressing stage (stage II), recovery stage (stage III), and dissipation stage (stage IV). In addition, the present study observed a correlation between patients’ clinical status (fever and cough) and the CT stages. Fourteen of the 19 patients who presented with fever as the initial symptom, experienced the disappearance of fever during the progressing stage (stage II), 4 patients experienced the disappearance of fever during the recovery stage (stage III), and 1 patients became fever free during the dissipation stage (stage IV), which may indicate that image change has some lag compared to fever change. All 6 patients presenting with a cough as the initial symptom experienced a disappearance of their cough during the dissipation stage (stage IV). The longer duration of the cough may be associated with the stimulation of residual inflammation. These findings indicate that the imaging stages based on the CT results correlate with clinical status and may aid in assessing disease progression and in adjusting treatment strategies in a timely manner.
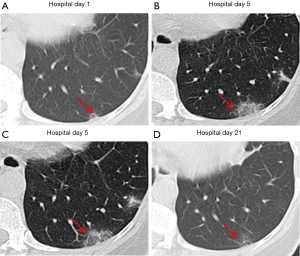
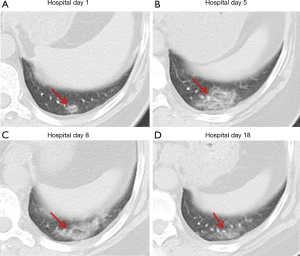
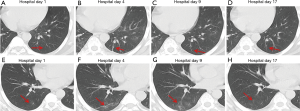
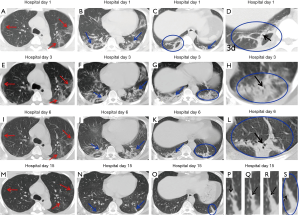
In a previous longitudinal study of CT images of SARS cases (13), the consolidation could transform into GGO or disappear, whereas GGO could persist or even progress to lobular septum thickening and fibrosis. This study found that in some COVID-19 patients, lesions in the bilateral lungs could also evolve into fibrosis-like stripes in a short period of time. However, the exact imaging pathological mechanism of this sign is currently unclear. Most radiologists in China believe it is a pulmonary interstitial stripe-shaped fibrotic lesion, and it may be a feature of the reversal of COVID-19 (14). Similar to COVID-19, SARS and MERS also often have pulmonary fibrosis. Some researchers have reported that the incidence of SARS-associated pulmonary fibrosis can be as high as 36.7–40% (15-17). Cao et al. autopsied patients who died of SARS and found that typical pulmonary fibrosis was detected 33 days after infection with SARS-CoV (18). A CT follow-up study by Zhang et al. also showed that pulmonary fibrosis in SARS patients first appeared at least 30 days after onset of the disease (19). Chen et al. and Lu et al. (20,21) found that stripe-shaped fibrosis lesions of varying degrees remained in SARS patients 1–12 months after the lesions were absorbed. Das et al. also found that 33% of patients with MERS still had stripe-shaped fibrosis lesions 1–7 months after recovery (22). A total of 8 cases of this group of patients had fibrosis-like stripes during the course of the disease. Four of these cases were identified at the first CT examination on admission, 3 cases were identified at the second CT examination, and 1 case was identified at the third CT examination. The fibrosis-like stripes were all located in the lower lobe (8/8, 100%), and mostly involved the bilateral lungs (7/8, 87.5%) (http://cdn.amegroups.cn/static/application/f60ac1df948fd97212228bd6cf343dbe.docx). The median time for the appearance of the fibrosis-like stripes was 4.5 days (1–8 days) after the onset of the disease, and in most cases (7/8, 87.5%) the stripes disappeared within a short period of time (median: 13 days, range: 9–20 days). Compared with the reported fibrosis lesions related to SARS (18-21) and MERS (22), the fibrosis-like stripes of the COVID-19 patients in this study have the characteristics of early appearance, rapid absorption, and rapid morphological changes. Therefore, we infer that the fibrosis-like stripes of COVID-19 may not be fibrosis lesions. Analysis of the CT signs of four consecutive chest CTs of case #3 (Figure 2) showed the following observations. The first CT showed consolidation of the lower lobe of the bilateral lungs and bronchial shadows (black arrow) in the consolidated lung tissue (Figure 9C,D), suggesting inflammation of the lower lobe of the bilateral lungs with local atelectasis. The second CT showed that the consolidation in the lower lobe of the bilateral lungs larger than before, with bronchial shadows (black arrow) in the consolidated lung tissue (Figure 9G,H). The third CT showed that the consolidation in the lower lobe of the bilateral lungs had reduced in size, but still had bronchial shadows (black arrow) in the consolidated lung tissue (Figure 9K,L), indicating that the inflammation was gradually absorbed, and the atelectasis of the lower lobe of bilateral lungs was gradually recovering. The fourth CT showed that only a few fibrosis-like stripes were left in the lower lobe of the bilateral lungs (Figure 9O). If dynamic CT examinations were not performed in this case, then it would be easy to mistake the small stripe-like shadows in the left lower lobe as fibrosis lesions based on the fourth CT image. However, when the images of the continuous layers of this lesion (Figure 9P,Q,R,S) were magnified, it revealed that there were still small bronchial shadows (black arrow) within the lesion, which when combined with the history of the gradual reduction of the atelectasis on the previous three CT examinations, suggests that this type of fibrosis-like stripe is a sub-segmental atelectasis rather than a fibrosis lesion. Therefore, we speculate that this fibrosis-like stripe is a temporary CT manifestation during the evolution of atelectasis in COVID-19, which may also explain why this fibrosis-like stripe has the characteristics of early appearance, rapid morphological change, and fast absorption. In addition, recent pathological evidence also showed the appearance of mucous plug in the bronchus in COVID-19 (23). We speculate that the mucous plug in the bronchus may be one possible reason for the atelectasis.
Nonetheless, as the number of cases exhibiting this typical evolving process is still small and the evidence is not sufficient. Accordingly, this hypothesis awaits further confirmation by large sample studies and more pathological studies.
This study has several advantages: (I) currently, systematic longitudinal imaging studies are urgently needed. In this study, we systematically explored the evolving patterns of chest CT characteristics of COVID-19, and our results are conducive to guiding clinical diagnosis and prognosis prediction. (II) Based on the dynamic change characteristics of patients’ chest CTs, we performed imaging staging of COVID-19 and conducted preliminary research on the disease’s time points in each stage. The research results can help clinicians better judge disease progression and adjust treatment strategies. (III) In the present study, we propose a new hypothesis that fibrosis-like stripe is a sub-segmental atelectasis rather than a fibrosis lesion, and for the first time, we identify key imaging evidence to support the hypothesis. We think this finding may contribute to a better understanding of the pathogenesis of COVID-19. This study also has the following limitations: (I) this is a retrospective study, and potential selection bias and information bias are inevitable. However, as a real-world study, this study objectively reflects the changes of this disease and has clinical value. (II) This study is a single-center study based on data relating to COVID-19 patients in the Taizhou area. The included patients all had mild- or ordinary-type disease, lacking imaging data of severe and critically ill patients. Furthermore, the sample size (n=22) is relatively small, which may limit the generalization of the research results. (III) The timing of chest CT re-examination was decided mainly based on the empirical judgment of the clinicians on the severity of the disease and clinical efficacy. Therefore, consistency of the time interval of the CT re-examinations was difficult to achieve. Because of the rapid change in the condition of the diseased patients, some important signs might have been missed. (IV) Pathological evidence was lacking, so correlation analysis of imaging and pathology could not be performed.
Conclusions
COVID-19 has an acute onset, and the chest CT image manifestations are complicated with the main imaging manifestations of different types of GGO with or without lung consolidation in the subpleural region of the bilateral lungs. The CT features of lung lesions change rapidly. The lung lesions of mild and ordinary types of COVID-19 may significantly improve or disappear within a short period after active treatment, showing good overall prognosis. Moreover, fibrosis-like stripes may be a sign of atelectasis of sub-segment lung tissue of COVID-19 and may be a specific indicator of COVID-19.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1363
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1363
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-1363
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at http://dx.doi.org/10.21037/jtd-20-1363). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of Taizhou Enze Hospital. Because of the retrospective nature of the study, patient consent for inclusion was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020;382:1199-207. [Crossref] [PubMed]
- World Health Organization. Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. Available online: (Accessed on February 12, 2020).https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020
- Tian HY. 2019-nCoV: new challenges from coronavirus. Zhonghua Yu Fang Yi Xue Za Zhi 2020;54:235-8. [PubMed]
- World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available online: (Accessed on March 11, 2020).https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- Kanne JP, Chest CT. Findings in 2019 Novel Coronavirus (2019-nCoV) Infections from Wuhan, China: Key Points for the Radiologist. Radiology 2020;295:16-7. [Crossref] [PubMed]
- Lei J, Li J, Li X, et al. CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology 2020;295:18. [Crossref] [PubMed]
- Shi H, Han X, Zheng C. Evolution of CT Manifestations in a Patient Recovered from 2019 Novel Coronavirus (2019-nCoV) Pneumonia in Wuhan, China. Radiology 2020;295:20. [Crossref] [PubMed]
- Liu P, Tan XZ. 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology 2020;295:19. [Crossref] [PubMed]
- Fang Y, Zhang H, Xu Y, et al. CT Manifestations of Two Cases of 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology 2020;295:208-9. [Crossref] [PubMed]
- Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020;30:3306-9. [Crossref] [PubMed]
- Li Y, Xia L. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. AJR Am J Roentgenol 2020;214:1280-6. [Crossref] [PubMed]
- National Health Commission of the People’s Republic of China. Diagnosis and treatment protocols of pneumonia caused by a novel coronavirus (trial version 3). Available online: http://www.nhc.gov.cn/yzygj/s7653p/202001/f492c9153ea9437bb587ce2ffcbee1fa/files/39e7578d85964dbe81117736dd789d8f.pdf, Published on January 22, 2020. 2020.
- Wu X, Dong D, Ma D. Thin-Section Computed Tomography Manifestations During Convalescence and Long-Term Follow-Up of Patients with Severe Acute Respiratory Syndrome (SARS). Med Sci Monit 2016;22:2793-9. [Crossref] [PubMed]
- Chinese Society of Radiology. Radiological Diagnosis of New Coronavirus Infected Pneumonitis: Expert Recommendation from the Chinese society of Radiology (First edition). Chinese Journal of Radiology 2020;54:E001.
- Xu YD, Li Y, Liu X, et al. Clinical therapy of severe acute respiratory syndrome: 38 cases retrospective analysis. Chinese Critical Care Medicine 2003;15:343-5. [PubMed]
- Liu J, Jiang S, Chen B. Image appearances of severe acute respiratory syndrome (a preliminary study of 260 patients) Chin J Med Imaging Technol 2003;19:790-2.
- Joynt GM, Gregory EA, Philip L, et al. Late-stage adult respiratory distress syndrome caused by severe acute respiratory syndrome: abnormal findings at thin-section CT. Radiology 2004;230:339-46. [Crossref] [PubMed]
- Cao HJ, Wang DW, Li N, et al. Pathologic process of pulmonary fibrosis in severe acute respiratory syndrome: a preliminary report. Bull Acad Mil Med Sci 2006;30:501-4.
- Zhang L, Lang Z, Sun L. The study of pulmonary fibrosis in severe acute respiratory syndrome. Chin J Infect Dis 2005;23:46-8.
- Chen D, Li M, Li H. The discussion of thoracic imaging manifestations of SARS. Chinese Journal of Misdiagnostics 2003;3:1443-6.
- Lu P, Yang G, Yu W, et al. Imaging follow-up of SARS patients complicated with pulmonary fibrosis. Chin J Med Imaging Technol 2004;20:1901-3.
- Das KM, Lee EY, Singh R, et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging 2017;27:342-9. [Crossref] [PubMed]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref] [PubMed]


