
Seroradiologic prognostic evaluation of acute exacerbation in patients with idiopathic interstitial pneumonia: a retrospective observational study
Introduction
Idiopathic pulmonary fibrosis (IPF) is a fibrotic lung disease of unknown etiology and has a poor prognosis (1,2). It is characterized by progressive worsening of dyspnea and lung function; however, for unknown reasons, some patients with IPF experience rapid and often fatal disease progression (3-6). This type of rapid progression is known as acute exacerbations (AEs) of IPF (AE-IPF). AEs were first reported in IPF but have since been reported in other types of idiopathic interstitial pneumonias (IIPs) (7,8). We have recently reported the frequency of AE of IIP (AE-IIP) (8) and demonstrated similar survival rates in AE-IPF and AE of non-IPF IIP (9). AE of non-IPF interstitial lung diseases (ILDs), including non-IPF IIP, chronic hypersensitivity pneumonia, and collagen vascular disease associated-interstitial pneumonias, is an important healthcare issue (10).
A diagnosis of IPF (11), respiratory dysfunction (9) and use of corticosteroids (9) during periods of a stable state, a lower partial oxygen tension (PaO2)/fraction of inspired oxygen (FiO2) ratio (9), and higher lactate dehydrogenase and inflammatory parameters at the onset of AE-IPF (12) have been reported to be significantly poor prognostic factors (13). High-resolution computed tomography (HRCT) findings are also an important predictor of the prognosis of AE-IPF patients. Akira et al. classified HRCT findings at the onset of AE-IPF into three patterns, i.e., peripheral, multifocal, or diffuse, and reported a worse prognosis in AE-IPF patients with a diffuse pattern than in those with a peripheral or multifocal pattern (14). They found that a radiologically diffuse pattern suggested a pathologic outcome of diffuse alveolar damage (DAD) and that a peripheral pattern was suggestive of organizing pneumonia (OP) (14).
Krebs von den Lungen (KL)-6 (15) and surfactant protein (SP)-D (16) are serum biomarkers of ILDs, and prognostic significance was suggested in IPF and the other IIPs. Furthermore, KL-6 can predict the occurrence of AE in IPF and IIPs (8,17) and is a significant prognostic factor in AE-IPF (18,19). We previously reported that SP-D is a significant prognostic factor in AE-IIPs (9). Although serum biomarker levels are often found to be elevated at the onset of AE compared to the levels recorded previously during a stable state, their prognostic significance and association with HRCT patterns of AE-IPF have not been examined. This clinical study aimed to answer these clinical questions in AE-IIP patients.
Methods
Study population
We retrospectively identified 113 patients with AE-IIPs diagnosed between 2004 and 2016 at Kinki-Chuo Chest Medical Center (KCCMC). Seventy-seven patients whose serum KL-6 and SP-D levels had been measured within the 6 months before and at the time of onset of AE were enrolled in the study (see Figure S1).
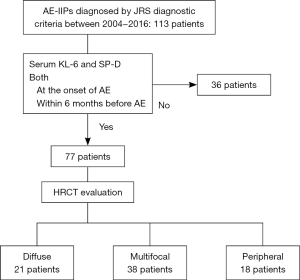
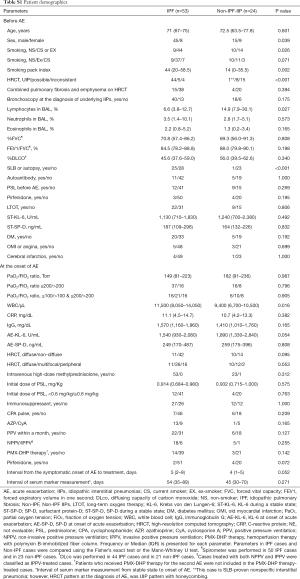
Clinical data for the 77 cases were collected from the medical records (Table 1). Sixty of the patients were male and the median age was 72 years. The underlying IIP was IPF in 53 cases and non-IPF in 24. Twenty-one patients were treated with prednisolone before the onset of AE and long-term oxygen therapy (LTOT) had been initiated in 31 cases.

Full table
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by our institutional review board (approval number 650, July 23, 2018). The need for patient consent was waived in view of the retrospective nature of the study and the anonymity of the data.
Diagnosis of underlying IIPs
Bronchoalveolar lavage (BAL) and/or transbronchial lung biopsy (TBLB) was performed while diagnosing IIP in 58 of the 77 cases. Twenty-five surgical lung biopsy (SLB) specimens and one autopsy specimen were pathologically evaluated. Underlying IPF was diagnosed based on the American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society (JRS)/Latin American Thoracic Association (ALAT) guideline for diagnosis and management of IPF (2). The IPF group included 53 patients, 24 of whom had SLB-proven idiopathic usual interstitial pneumonia (UIP) and 1 had autopsy-proven UIP. They were classified into definite (n=20), possible IPF (n=5), and clinically diagnosed IPF cases (n=28) with UIP HRCT pattern. The remaining 24 patients comprised the non-IPF IIP group (n=24); one patient was diagnosed with nonspecific interstitial pneumonia by SLB (20), and the remaining 23 had unclassifiable IIP without SLB evaluation and did not have cryptogenic OP after clinical evaluation with (n=17) or without (n=6) bronchoscopic findings. Our cohort did not include patients with collagen vascular disease associated-interstitial pneumonia diagnosed using specific diagnostic criteria. Serum autoantibody positivity was evaluated based on the criterion of “interstitial pneumonia with autoimmune features (IPAF)” in the serological domain (21). Anti-neutrophil cytoplasmic antibody levels were also evaluated. Of 16 patients with positive autoantibodies, one showed Reynaud’s phenomenon and satisfied the IPAF criteria. Chronic hypersensitivity pneumonia was ruled out based on clinical and bronchoscopic findings. Percentages of lymphocytes in BAL were less than 40%, except in two cases (22). Granulomatous lesions were not detected on TBLB specimens. HRCT did not show prominent mosaic attenuation (22).
An expert in diffuse lung diseases (TA) and a chest radiologist (MA) retrospectively reviewed the de-identified HRCT films obtained before the onset of AE and classified them into a UIP pattern (n=45), a possible UIP pattern (n=13), or inconsistent with a UIP pattern (n=19) according to the guideline’s criteria (2). All patients with possible UIP patterns had traction bronchiectasis. Emphysema was diagnosed based on Schmidt et al.’s HRCT scan criteria (23). Patients complicated with moderate or severe emphysema were described as having combined pulmonary fibrosis and emphysema.
Diagnosis and treatment of AE-IIPs
AE-IIPs were diagnosed according to the modified Japanese Respiratory Society (24) criteria for AE-IPF (Supplementary File). Apparent infection was excluded; however, AE-IIPs triggered by infection may have been included because endotracheal aspiration or bronchoalveolar lavage was performed in 12 patients. SLB was not performed for the diagnosis of AE-IIPs. The AE-IIPs were generally treated with prednisolone following intravenous administration of methylprednisolone for three consecutive days with/without an immunosuppressant (9) and treatment in detail was shown in Table 1.
HRCT findings at diagnosis of AE-IIPs
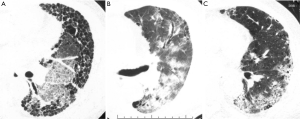
The HRCT pattern at the onset of AE was classified by three chest radiologists (MA, TO, SS) as peripheral, multifocal, or diffuse (14) (see Figure S2). The HRCT pattern was then classified as diffuse or non-diffuse (peripheral or multifocal).
Measurement of serum markers and related parameters
We measured serum markers, including KL-6 and SP-D, using commercially available enzyme-linked immunosorbent assay (ELISA) kits, i.e., ED046 (Eizai, Tokyo, Japan) and SP-D ELISA (Yamasa, Tokyo, Japan) using cut-off levels of 500 U/mL and 110 ng/mL, respectively (25). These markers were measured both at the onset of AE (AE-KL-6 and AE-SP-D) and within the 6 months before AE when the disease was stable (ST-KL-6 and ST-SP-D). The measurement interval between stable state and AE onset was 50 days (median) and less than 90 days in 62 cases (80.5%). We compared the increase in serum levels of these markers (ΔKL-6 and ΔSP-D) and the rate of increase in serum markers when compared with the stable state (ΔKL-6/ST-KL-6 and ΔSP-D/ST-SP-D). Median values of ΔKL-6/ST-KL-6 and ΔSP-D/ST-SP-D were 0.211 and 0.410, respectively, and the two parameters were categorized into a higher and a lower group by their median values.
Statistical analysis
Continuous variables were presented as medians with interquartile range. We compared all parameters in the AE-IIP cases with the three HRCT patterns using the Kruskal-Wallis test, Mann-Whitney U test or Fisher’s exact test. Kaplan-Meier analysis and Wilcoxon test were used to examine survival. Univariate Cox proportional hazards regression analysis was used to calculate the hazard ratio for each parameter and predict survival after AE. Multi-collinearity of each parameter was examined by correlating the regression coefficient calculated by multivariate Cox proportional hazard regression analysis. If absolute values of correlation coefficients of two parameters were more than 0.7, one of the parameters with a higher P value for hazard ratio was excluded from the multivariate analysis. Spearman’s rank correlation was additionally performed for all the parameters. If the correlation of two parameters was significant (P<0.05) with rho >0.7, one of the parameters with a higher P value for hazard ratio were excluded from the multivariate analysis. Using the remaining parameters, prognostic factors in AE-IIP patients were determined by multivariate analysis with a stepwise selection procedure. All analyses were performed in all patients and separately for patients with AE-IIP according to whether the HRCT pattern was peripheral, multifocal, or diffuse. Statistical significance was inferred at P<0.05. All statistical analyses were performed using SPSS v. 24 for Macintosh software (IBM Corp., Armonk, NY, USA).
Results
Patient demographics
There was no significant difference in survival between patients with AE-IPF and those with AE of non-IPF IIP (P=0.875, log-rank test). Furthermore, the clinical parameters in patients with AE-IPF were similar to those in patients with AE of non-IPF IIP, except for patient sex, smoking history, smoking pack index, and percentages of lymphocytes in BAL (see Table S1). Hence, the analysis was performed for all AE-IIP cases.
Full table
The patient demographics are shown in Table 1. The median age at the diagnosis of AE-IIP was 72 years and 60 patients were male. The median survival time (MST) in all cases was 61 days. Serum levels of KL-6 (P<0.001) and SP-D (P<0.001) at the time of AE increased significantly when compared with the stable state before AE (Wilcoxon signed-rank test).
HRCT pattern and prognosis
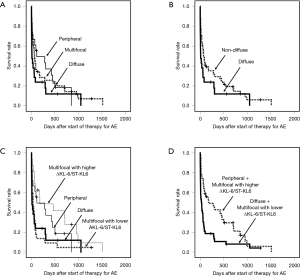
The HRCT pattern for AE-IIP cases was diffuse (n=21), multifocal (n=38), or peripheral (n=18). The MST in patients with AE-IIP and a diffuse pattern was significantly worse than that in those with a peripheral pattern (12 vs. 115 days, P=0.017) and tended to be worse than in those with a multifocal pattern (12 vs. 67 days, P=0.059; Figure 1A). MST in patients with AE-IIP and a diffuse pattern was worse than that in those with a non-diffuse pattern (n=56, 73 days), including both peripheral and multifocal patterns, (P=0.014, Wilcoxon test; Figure 1B).
Differences in clinical features according to HRCT pattern
The white blood cell, lactate dehydrogenase, ΔKL-6, AE-SP-D, ΔSP-D, and ΔSP-D/ST-SP-D values were significantly higher in patients with the diffuse pattern than in those with a multifocal pattern (Table 2, Mann-Whitney U test). The PaO2/FiO2 ratio also tended to be lower when the pattern was diffuse than when it was multifocal. The clinical features and treatment before and at the onset of AE were identical in AE-IIPs with a multifocal or peripheral pattern (Table 2).

Full table
Prognostic factors according to HRCT pattern
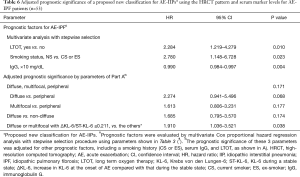
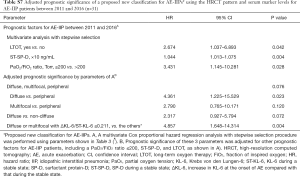
Prognostic factors in all patients were evaluated by univariate Cox proportional hazard regression analysis (Table 3). Using the parameters remaining after exclusion due to multi-collinearity evaluation (see Table S2) and Spearman’s rank correlation (see Table S3), multivariate Cox proportional hazard regression analysis with the stepwise selection method was performed for all cases and separately for the three HRCT patterns (Table 4). In all cases, implementation of LTOT before AE, a higher ST-SP-D, and a PaO2/FiO2 ratio ≤200 Torr were significantly poor prognostic factors in multivariate analysis. Multivariate analysis revealed a higher AE-KL-6 in the presence of a peripheral pattern, a PaO2/FiO2 ratio ≤200 Torr, a lower ΔKL-6/ST-KL-6 value (≤0.211) in the presence of a multifocal pattern, and a higher ST-SP-D value in the presence of a diffuse pattern as significantly poor prognostic factors.
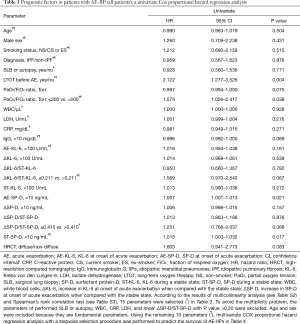
Full table
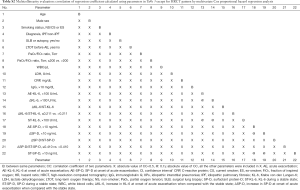
Full table
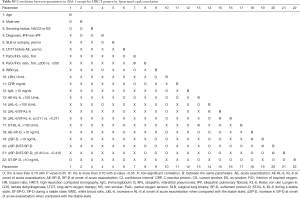
Full table
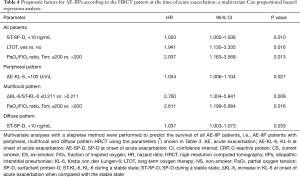
Full table
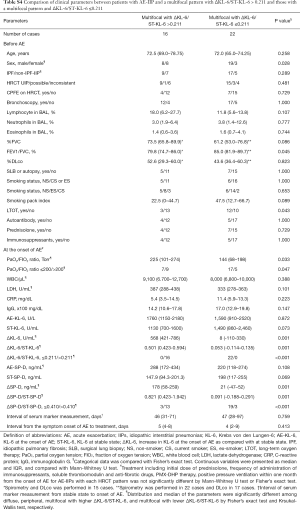
Classification of AE-IIP patients with HRCT pattern and ΔKL-6/ST-KL-6
We have classified AE-IIPs into four groups; diffuse pattern, peripheral pattern, multifocal pattern with lower ΔKL-6/ST-KL-6 (≤0.211), and multifocal pattern with higher ΔKL-6/ST-KL-6 (>0.211). Survival with a multifocal pattern and lower ΔKL-6/ST-KL-6 (≤0.211) was significantly worse than with a higher ΔKL-6/ST-KL-6 (>0.211) (Figure 1C, P=0.002, Wilcoxon test). There was no significant difference between the diffuse pattern and multifocal pattern with ΔKL-6/ST-KL-6 ≤0.211 (P=0.689) or between the peripheral pattern and multifocal pattern with ΔKL-6/ST-KL-6 >0.211 (P=0.279).
Difference in clinical features between multifocal patterns with higher and lower ΔKL-6/ST-KL-6
In a multifocal pattern with lower ΔKL-6/ST-KL-6 (≤0.211), the ΔKL-6, ΔSP-D, ΔSP-D/ST-SPD and PaO2/FiO2 ratio were significantly lower and the frequency of LTOT was significantly higher than that with a higher ΔKL-6/ST-KL-6 (see Table S4). ST-KL-6 and ST-SP-D in multifocal pattern with lower ΔKL-6/ST-KL-6 tended to be higher than that with higher ΔKL-6/ST-KL-6 (see Table S4).
Full table
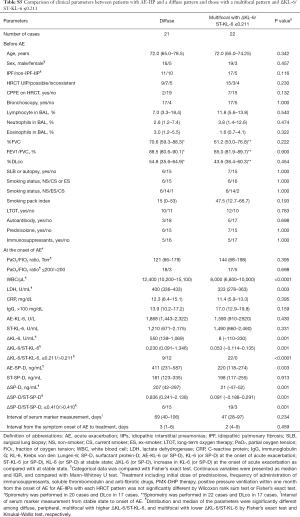
Difference in clinical features between the diffuse pattern and multifocal pattern with lower ΔKL-6/ST-KL-6
Background parameters before AE and PaO2/FIO2 ratio at the onset of AE were similar in the two groups. White blood cells, lactate dehydrogenase, ΔKL-6, ΔKL-6/ST-KL-6, AE-SP-D, ΔSP-D, and ΔSP-D/ST-SPD of the diffuse pattern was significantly higher than those of the multifocal pattern with lower ΔKL-6/ST-KL-6 (see Table S5).
Full table
Difference in clinical features between peripheral pattern and multifocal patterns with higher ΔKL-6/ST-KL-6
Background parameters except for sex were similar in both groups (see Table S6). Multifocal patterns with higher ΔKL-6/ST-KL-6 showed a higher ΔKL-6, more frequently a higher ΔKL-6/ST-KL-6 (>0.211), and a lower ST-SP-D than peripheral patterns (see Table S6).

Full table
Proposed classification of AE-IIPs using HRCT pattern and ΔKL-6/ST-KL-6
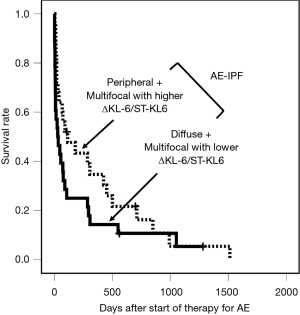
We divided the AE-IIPs into two groups, i.e., a group with a diffuse or multifocal pattern and a lower ΔKL-6/ST-KL-6 and a group with a peripheral or multifocal pattern and a higher ΔKL-6/ST-KL-6 (Figure 1D). Wilcoxon test revealed the significant survival difference between the two groups (P<0.0001, Figure 1D). After adjustment for other prognostic factors, a diffuse or multifocal pattern with a lower ΔKL-6/ST-KL-6 was a significantly poor prognostic factor (Table 5), although a diffuse pattern alone was not. Similarly, none of the three patterns seen on HRCT were significant prognostic factors.

Full table
Our proposed classification of AE-IIP patients can predict the survival of AE-IPF (n=53) (Table 6) after adjustment for other prognostic factors, although univariate analysis cannot show the significant survival difference (P=0.057, Wilcoxon test) (Figure 2). The classification can also predict survival AE-IIP patients who were diagnosed between 2011 and 2016 (n=31) (see Table S7).
Full table
Full table
Discussion
In this study, we demonstrated that AE-IIP patients with a multifocal HRCT pattern and lower ΔKL-6/ST-KL-6 value have a worse prognosis than their counterparts with a higher ΔKL-6/ST-KL-6 after adjusting for other prognostic factors; however, the prognosis in both groups is similar if the HRCT pattern is diffuse or peripheral. A multifocal pattern with a lower ΔKL-6/ST-KL-6 value might correspond histologically to a DAD pattern and that with a higher ΔKL-6/ST-KL-6 to an OP pattern. Therefore, we can divide AE-IIPs into two populations, i.e., according to the possibility of DAD or OP, using the combined criteria of HRCT pattern and the ΔKL-6/ST-KL-6 value, given that the prognosis of the two populations is significantly different after adjusting for other prognostic factors.
The main histologic pattern of AE-IPF is DAD (2,6,26). Previous studies using SLB showed various frequencies of an OP pattern superimposed on a UIP pattern (27,28). SLB-proven OP in AE-IPF was associated with better survival in both these reports. However, radiologic findings might be more important than histologic findings in general clinical settings when diagnosing AE-IPF and predicting patient survival because morphological overlaps between organizing DAD and OP (29) and sampling errors due to the patchy nature of acute lesions in AE-IPF (27) were reported.
Akira et al. were the first to identify the importance of the HRCT pattern when predicting the prognosis of AE-IPF (4,14). Survival was poor in their patients with AE-IPF and a diffuse pattern, which typically corresponds with a pathologic pattern of DAD, and good in those with a peripheral pattern, which usually corresponds with a pathologic pattern of OP (4). There are reports for (30) and against (18) the importance of HRCT patterns in predicting the survival of AE-IPF patients. Silva et al. reported histological and radiological discrepancies (28). This may reflect demographic differences in patients with the multifocal AE-IPF pattern, as seen in our study.
In a retrospective study on 58 AE-IPF patients, Akira et al. found serial changes from multifocal to diffuse pattern (14). They obtained autopsy specimens from 20 diffuse patients and 3 multifocal patients, all of which showed DAD with UIP (14). However, in an earlier study by the same researchers noted that 3 of 6 patients with AE-IPF and a multifocal pattern improved after high-dose corticosteroid therapy (4). Patients with corticosteroid-responsive AE-IIP and a multifocal pattern might show a pathologic OP pattern. We suspect that patients with a multifocal pattern constitute a heterogeneous group, possibly with pathologic findings of OP and DAD (4,14). This hypothesis is consistent with the radiologic observation that both cryptogenic OP (31) and acute interstitial pneumonia (32) may show a patchy distribution.
Patients with acute respiratory distress syndrome (ARDS) usually show a DAD histologic pattern and mildly elevated serum KL-6 levels (33) that gradually increase as the illness progresses (34). Nathani et al. (33) reported a median KL-6 value of 422 U/mL at onset of ARDS and 588 U/mL 3 days later. Kondo et al. reported similar KL-6 levels at the onset of ARDS (34). We suggest that these values might be smaller than expected. However, peak serum KL-6 levels increased to a mean of 1,060.8 U/mL in non-survivors (34) and the elevation of serum KL-6 levels from the baseline to the peak was about 500 U/mL. The increase in KL-6 among non-survivors of ARDS is similar to the median ΔKL-6 value (550 U/mL) in our diffuse AE-IIP patients, whose prognosis was mostly poor. We could not find any other studies that measured ΔKL-6 levels in AE-IIP patients. However, Yokoyama et al. reported an elevated KL-6 value at the time of AE-IIP diagnosis and its subsequent increase, especially in patients who were non-responsive to steroids (35).
Akira et al. reported that patients with multifocal patterns may show a pathologic DAD pattern or may progress to a diffuse pattern (14). If a multifocal pattern with a lower ΔKL-6/ST-KL-6 value indicated an early diffuse pattern, the ΔKL-6 and ΔKL-6/ST-KL-6 values in the diffuse pattern would be expected to be significantly larger than those in a multifocal pattern with a lower ΔKL-6/ST-KL-6, because serum KL-6 levels gradually increase with progression of AE-IIP, and this hypothesis would be consistent with our data (see Table S5). Therefore, a multifocal pattern with a lower ΔKL-6/ST-KL-6 value might indicate an early diffuse pattern and suggest a pathologic DAD pattern. The median ΔKL-6 value in AE-IIP with a multifocal pattern and a lower ΔKL-6/ST-KL-6 was 8 U/mL; this figure is consistent with that of previous reports of serum KL-6 levels at the time of onset of ARDS being almost within the normal range (33,34).
In our study, a higher AE-KL-6 in AE-IIPs with a peripheral pattern suggested a poor prognosis. Okada et al. (36) reported that cryptogenic OP with an elevated KL-6 level was suggestive of the presence of traction bronchiectasis and distortion. We found that a higher AE-KL-6 value was a significantly poor prognostic factor for peripheral pattern AE-IIP, which is consistent with Okada et al.’s report (36). ΔSP-D/ST-SP-D and ΔSP-D of AE-IIPs with a multifocal pattern and higher ΔKL-6/ST-KL-6, reflecting newly appeared acute lesions, was significantly higher than that of AE-IIPs with a multifocal pattern and a lower ΔKL-6/ST-KL-6 (both P=0.001; Table S4). These results are consistent with the positive correlation found between serum KL-6 and SP-D levels in patients with cryptogenic OP reported by Yamagishi et al. (37) and the hypothesis that pathologic OP has a multifocal pattern with higher ΔKL-6/ST-KL-6.
SP-D is produced by hyperplastic epithelial cells (38). Patients with IIP and a higher ST-SP-D may have more such cells that are vulnerable to extensive injury by various mechanisms, which would put them at increased risk of a severe AE. This hypothesis is consistent with our finding that a higher ST-SP-D in patients with the diffuse pattern can predict a poor prognosis. ST-SP-D of multifocal pattern and lower ΔKL-6/ST-KL-6, suggesting histological DAD, tended to be higher than that of multifocal pattern and higher ΔKL-6/ST-KL-6. Hence, higher ST-SP-D might suggest presence of increased baseline alveolar injury and future occurrence of DAD-type AE.
This study has some limitations. First, it had a retrospective single-center design and included a limited number of patients. Therefore, our results and our proposed classification need to be validated in cohort studies in the future; however, the classification was also a significant prognostic factor for subgroups of our subjects; AE-IPF and AE-IIP diagnosed between 2011 and 2016. Second, the interval between measurements of serum markers in the stable state and those obtained at the time of AE varied from patient to patient; however, in about 80% of cases, the interval was less than 3 months. Third, the link between our proposed classification and pathologic findings was not confirmed by the findings on SLB. Fourth, KL-6 cannot be measured in all countries, so use of more universally available clinical markers to classify AE-IIP with a multifocal pattern would be expected. Fifth, the possibility that we included AE patients with a disease other than an IIP cannot be excluded because most non-IPF IIP cases were diagnosed without SLB. However, the autoantibody positivity rate and HRCT pattern of the underlying IIPs (UIP, possible UIP, inconsistent with UIP) were not significantly different between the three HRCT patterns at the time of AE (Table 2). Furthermore, Suzuki et al. reported that the prognosis of AE-IPF was similar to that of AE of non-IPF ILDs (39) and that the prognosis of AEs of all types of ILDs could be evaluated together.
Conclusions
Combining the HRCT pattern and the ΔKL-6/ST-KL-6 value can improve our ability to predict the survival of AE-IIP patients. We hope that our classification will be evaluated by other investigators in the future.
Supplementary
Between 2004 and 2016, we have diagnosed 113 patients with AE-IIP according to the diagnostic criteria of the Japanese Respiratory Society (JRS). AE-IIP patients, whose serum levels of KL-6 and SP-D was evaluated both at the onset of AE and within 6 months before the onset of AE, was selected and 77 patients were enrolled in this study. High-resolution computed tomography (HRCT) films at the onset of AE was classified into 3 patterns (diffuse, multifocal and peripheral) according to the criteria of Akira (14).
Diagnostic criteria of acute exacerbation (AE) in Idiopathic interstitial pneumonias (IIPs)
AE-IIPs were diagnosed according to the modified AE-IPF criteria of the Japanese Respiratory Society (25). (I) Within one month, the following three conditions were all satisfied during the disease progression of IIPs: (i) progressively worsening dyspnea; (ii) new ground-glass attenuation (GGA) evident in HRCT superimposed on a background reticular or honeycomb pattern; and (iii) a reduction in PaO2 at rest of more than 10 Torr relative to previous measurements. (II) Exclusion of obvious causes of acutely impaired respiratory function, such as infection, pneumothorax, cancer, pulmonary embolism, or congestive cardiac failure. Apparent infections were carefully excluded by measuring antibodies for Mycoplasma pneumoniae and Chlamydia pneumoniae in paired sera, β-D glucan, Cytomegalovirus antigen, and bacterial cultures of blood and sputum.
We evaluated the HRCT pattern at the onset of AE according to the classification of Akira (14). For a diffuse pattern (Figure S2A), the ground glass attenuation (GGA) was homogeneously distributed. For the multifocal pattern (Figure S2B), parenchymal opacification was apparent in both the central and peripheral regions. For the peripheral pattern (Figure S2C), parenchymal opacification appeared in the inner peripheral region adjacent to pre-existing subpleural honeycombing or peripheral interstitial opacity.
Acknowledgments
We would like to thank Masaki Hirose for the statistical discussion, Ms. Yuki Matsui for secretarial support, and Editage (www.editage.com) and Uni-Edit (www.uni-edit.net) for English language editing.
Funding: This study was partially supported by a grant from the National Hospital Organization {H28-NHO(Kokyu)-2, H26-NHO(Kokyu)-01, H22-NHO(Kokyu)-2-16} that was awarded to TA and YI, and AMED: DLD/14526278 and PAP/14526182, which was awarded to YI individually and to YI and TA, respectively.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-911
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-911
Availability of Data and Material: The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Conflicts of Interest: All authors have completed the ICMJR uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-911). YI reports grants from National Hospital Organization, grants from Japanese Ministry of Health, Labour, and Welfare, grants from Japan Agency for Medical Research and Development, during the conduct of the study; other from Boehringer Ingelheim, other from Shionogi and co. ltd, other from Asahi Kasei, outside the submitted work. TA reports grants from National Hospital Organization, grants from Japan Agency for Medical Research and Development, during the conduct of the study; personal fees from Boehringer Ingelheim, personal fees from Shionogi and co. ltd, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the institutional review board of Kinki-Chuo Chest Medical Center (approval number 650, acceptance date: July 23, 2018). The need for patient consent was waived in view of the retrospective nature of the study and the anonymity of the data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- King TE Jr. Idiopathic pulmonary fibrosis. Lancet 2011;378:1949-61. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Kondoh Y, Taniguchi H, Kawabata Y, et al. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest 1993;103:1808-12. [Crossref] [PubMed]
- Akira M, Hamada H, Sakatani M, et al. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol 1997;168:79-83. [Crossref] [PubMed]
- Collard HR, Moore BB, Flaherty KR, et al. with the Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636-43. [Crossref] [PubMed]
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 2007;132:214-20. [Crossref] [PubMed]
- Arai T, Kagawa T, Sasaki Y, et al. Heterogeneity of incidence and outcome of acute exacerbation in idiopathic interstitial pneumonia. Respirology 2016;21:1431-7. [Crossref] [PubMed]
- Arai T, Tachibana K, Sugimoto C, et al. High-dose prednisolone after intravenous methyl prednisolone improves prognosis of acute exacerbation in idiopathic interstitial pneumonias. Respirology 2017;22:1363-70. [Crossref] [PubMed]
- Kolb M, Bondue B, Pesci A, et al. Acute exacerbation of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180071. [Crossref] [PubMed]
- Tachikawa R, Tomii K, Ueda H, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration 2012;83:20-7. [Crossref] [PubMed]
- Song JW, Hong SB, Lim C-M, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356-63. [Crossref] [PubMed]
- Kondoh Y, Cottin V, Brown KK. Recent lessons learned in the management of acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir Rev 2017;26:170050. [Crossref] [PubMed]
- Akira M, Kozuka T, Yamamoto S, et al. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008;178:372-8. [Crossref] [PubMed]
- Ishikawa N, Hattori N, Yokoyama A, et al. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig 2012;50:3-13. [Crossref] [PubMed]
- Takahashi H, Shiratori M, Kanai A, et al. Monitoring markers of disease activity for interstitial lung diseases with serum surfactant proteins A and D. Respirology 2006;11:S51-4. [Crossref] [PubMed]
- Ohshimo S, Ishilawa N, Horimasu Y, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med 2014;108:1031-9. [Crossref] [PubMed]
- Fujimoto K, Taniguchi H, Johkoh T, et al. Acute exacerbation of idiopathic pulmonary fibrosis: high-resolution CT scores predict mortality. Eur Radiol 2012;22:83-92. [Crossref] [PubMed]
- Kishaba T, Tamaki H, Shimaoka Y, et al. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung 2014;192:141-9. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansel DM, et al. Official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Fischer A, Antoniou KM, Brown KK, et al. on behalf of the “ERS/ATS Task Force on Undifferentiated Forms of CTD-ILD”. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015;46:976-87. [Crossref] [PubMed]
- Morisset J, Johannson KA, Jones KD, et al. Identification of diagnostic criteria for chronic hypersensitivity pneumonitis. An international modified Delphi survey. Am J Respir Crit Care Med 2018;197:1036-44. [Crossref] [PubMed]
- Schmidt SL, Nambiar AM, Tayob N, et al. Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema. Eur Respir J 2011;38:176-83. [Crossref] [PubMed]
- Taniguchi H, Ebina Y, Kondoh T, et al. Pirfenidone Clinical Study Group in Japan. Pirfenidone in idiopathic pulmonary fibrosis. Online supplement. Eur Respir J 2010;35:821-9. [Crossref] [PubMed]
- Inoue Y, Trapnell BC, Tazawa R, et al. Japanese Center of the Rare Lung Diseases Consortium. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med 2008;177:752-62. [Crossref] [PubMed]
- Oda K, Ishimoto H, Yamada S, et al. Autopsy analyses in acute exacerbation of idiopathic pulmonary fibrosis. Respir Res 2014;15:109. [Crossref] [PubMed]
- Churg A, Müller NL, Silva CI, et al. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol 2007;31:277-84. [Crossref] [PubMed]
- Silva CI, Müller NL, Fujimoto K, et al. Acute exacerbation of chronic interstitial pneumonia: high-resolution computed tomography and pathologic findings. J Thorac Imaging 2007;22:221-9. [Crossref] [PubMed]
- Kim DS, Park JH, Park BK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143-50. [Crossref] [PubMed]
- Usui Y, Kaga A, Sakai F, et al. A cohort of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ Open 2013;3:e002971. [Crossref] [PubMed]
- Lee JW, Lee KS, Lee HY, et al. Cryptogenic organizing pneumonia: serial high-resolution CT findings in 22 patients. AJR Am J Roentgenol 2010;195:916-22. [Crossref] [PubMed]
- Ichikado K, Johkoh T, Ikezoe J, et al. Acute Interstitial Pneumonia: High-Resolution CT findings correlated with pathology. AJR Am J Roentgenol 1997;168:333-8. [Crossref] [PubMed]
- Nathani N, Perkins GD, Tunnicliffe W, et al. Krebs von den Lungen 6 antigen is a marker of alveolar inflammation but not of infection in patients with acute respiratory distress syndrome. Crit Care 2008;12:R12. [Crossref] [PubMed]
- Kondo T, Hattori N, Ishikawa N, et al. KL-6 concentration in pulmonary epithelial lining fluid is a useful prognostic indicator in patients with acute respiratory distress syndrome. Respir Res 2011;12:32. [Crossref] [PubMed]
- Yokoyama A, Kohno H, Hamada H, et al. Circulating KL-6 predicts the outcome of rapidly progressive idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;158:1680-4. [Crossref] [PubMed]
- Okada F, Ando Y, Honda K, et al. Comparison of pulmonary CT findings and serum KL-6 levels in patients with cryptogenic organizing pneumonia. Br J Radiol 2009;82:212-8. [Crossref] [PubMed]
- Yamagishi T, Kodaka N, Watanabe K, et al. A retrospective clinical research of relapse organizing pneumonia. Ann Thorac Med 2020;15:15-20. [Crossref] [PubMed]
- Pan T, Nielsen L, Allen MJ, et al. Serum SP-D is a marker of lung injury in rats. Am J Physiol Lung Cell Mol Physiol 2002;282:L824-L832. [Crossref] [PubMed]
- Suzuki A, Kondoh Y, Brown KK, et al. Acute exacerbation of fibrotic interstitial lung diseases. Respirology 2020;25:525-34. [Crossref] [PubMed]


