
Gender effects on quality of life and symptom burden in patients with lung cancer: results from a prospective, cross-cultural, multi-center study
Introduction
Gender aspects are becoming an increasingly important topic in the treatment of lung diseases. A number of recent clinical examples illustrate this point. Lung cancer, one of the most common cancers worldwide and the leading cause of cancer associated with death (1-3), has witnessed a growing incidence in women in recent years (1,4-6). Female patients present with more advanced disease stages and at a younger age than men, which may be attributable to time trends in smoking habits and their related effects (1). Adenocarcinoma is the most common type of lung cancer in female patients (1,7). Women respond better to various therapies in non-small cell lung cancer (NSCLC) than men with benefits in overall survival (1,5). A Japanese nationwide registry study covering 12,509 patients showed overall survival benefits for women after resection of primary lung neoplasms (5-year survival rate of women 75.6% vs. men 57.95%) (7). Women with chronic obstructive pulmonary disease (COPD) suffer from more exacerbations (8) and greater impairment of their health status than men (9). A recent review on asthma coined the phrase “the female lung” and came to the conclusion that women experience more asthma symptoms than men and use more rescue medications, which results in reduced quality of life (QoL) (10).
The analysis of gender differences has a long tradition in QoL research (11-17). Large-scale studies on representative samples conducted in Germany and Norway have consistently shown that women report lower levels of QoL and higher symptom burden than men as assessed by the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 core questionnaire (11-17). This gender effect is relevant with regard to oncological therapy, since considerations regarding patients’ QoL during and after treatment are becoming a topic of growing interest (13,18). For one, QoL contributes to increased overall survival (19), for the other improving QoL is a treatment goal on its own. In the context of clinical studies it has become standard to include QoL measures as endpoint variables (20). Therefore, for determining gender differences in patients with lung cancer is important to identify areas of need for patients and to ensure the best possible clinical outcome.
Lung cancer patients may suffer from numerous symptoms, such as dyspnea, coughing, pain and fatigue, which have a negative impact on their QoL (4,21). The EORTC QLQ-C30 is a well-known questionnaire used to assess QoL of cancer patients in oncological clinical trials (11). Additionally, as a lung cancer-specific module the QLQ-LC13 covers 13 typical symptoms of lung cancer patients. It has been used since 1994 together with the core questionnaire QLQ-C30 (22). Due to the changes in diagnostic and therapeutic options in lung cancer treatment, an international multicenter research project has been initiated to update the EORTC lung cancer module, according to the scientifically acknowledged EORTC procedure (20,22).
In the course of this project data are available that allow to investigate gender differences in QoL reporting. The goal of the present paper was to analyze whether female and male lung cancer patients differ with regard to their self-reported somatic symptoms and functional QoL outcomes, and whether certain symptoms are reported as being more intense or more frequent in women than in men. Based on the previous findings of large scale studies (11-17), we proceeded with the hypothesis that women report worse QoL and more intense symptoms than men. We present the following article in accordance with the SURGE reporting checklist (23) (available at http://dx.doi.org/10.21037/jtd-20-1054).
Methods
Study design
The current report is based on a prospective, international, cross-cultural, multicenter study that was designed to update the EORTC QLQ-LC13. Patients were stratified according to their primary therapy (surgery, radiochemotherapy or targeted therapy) and time frame (questionnaire administration during or shortly after therapy) in order to pick up side effects related to the therapy when assessing QoL. The study recruitment took place in nine different geographical locations (Germany, Great Britain, Italy, Israel, Norway, Poland, Spain, Taiwan and Cyprus) from February 2014 until February 2015. The study was approved by the Ethical Committee of the University of Regensburg, Germany (reference number 11-101-0024) and by the local ethical committees of the participating centers. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients gave their informed consent prior to being enrolled into this study. Details of the study methodology have been reported earlier (14). The original study protocol was registered with clinicaltrials.gov (reference number NCT01434784).
Patients
The following eligibility criteria applied: histologically proven non-small cell lung cancer (NCSLC) or small cell lung cancer (SCLC), 18 years of age or older, no previous other or recurrent tumor, cognitive and physical ability to fill in a questionnaire and written informed consent. Patients were excluded from the study if any of the above criteria was not fulfilled.
Procedure
Upon being informed about the study and providing written consent, patients filled in the paper-and-pencil version of the EORTC QLQ-C30 and the updated lung cancer module QLQ-LC29 (22). Thereafter, patients participated in a structured interview to evaluate the content of the questionnaires. Clinical data were recorded by the study personnel, using a standardized case report form (CRF).
Questionnaires
The EORTC QLQ-C30 (version 3.0) is a core questionnaire designed for the use in international clinical trials and addresses issues relevant for cancer patients of any tumor type (24,25). As its name suggests, the questionnaire consists of 30 individual items that are aggregated into a global quality of life score, five multi-item function scores (social, role, physical, cognitive, and emotional functioning), three multi-item symptom scores (nausea, pain, fatigue), and five single items (diarrhea, constipation, dyspnea, appetite loss, insomnia). Items are accompanied by four-item Likert scales with the response options labeled (I) “not at all”, (II) “a little”, (III) “quite a bit” and (IV) “very much”, with the exception of the two global quality of life items that are presented with a seven-item Likert scale (1=very poor to 7=excellent). According to the EORTC scoring manual, linear transformations are applied, resulting into scores from 0 to 100 (25). In the case of functional scores 0 denotes lowest and 100 highest functioning, in the case of symptom scales 0 denotes lowest and 100 highest symptom burden.
The updated EORTC lung cancer module consists of 29 items (22). The original QLQ-LC13 has been preserved (with the exception of one item tapping into pain medication) and amended by items assessing therapy-related side effects, existential issues and surgery-related symptoms. An initial psychometric analysis suggests that the QLQ-LC29 consists of five multi-item scales (coughing, shortness of breath, side effects, fear of progression, surgery-related symptoms) and five single items (coughing blood, pain in the chest, pain in the shoulder, bodily pain, problems with weight loss) (22).
Statistical analyses
Basic descriptive statistics included counts, percentages, medians/interquartile ranges (IQR), means and confidence intervals.
Gender differences in all QoL aspects were analyzed using univariate (t-test) and multivariable (analyses of covariance, ANCOVA) models. ANCOVAs adjusted for the following covariates: age, tumor type (NSCLC vs. SCLC), therapeutic approach (curative vs. palliative), living partner (with vs. without), education (compulsory vs. higher), primary therapy (surgery vs. chemo radiation therapy vs. targeted therapy), stage (NSCLC stage IV vs. other stages), region (English speaking vs. northern vs. southern vs. eastern countries) and comorbidity (yes/no). Estimated marginal means with corresponding 95%-confidence intervals were presented as effect estimates. A P value <0.05 was considered as the threshold of statistical significance. Due to the exploratory nature of all analyses, corrections for alpha-error were not applied. Furthermore, we compared descriptive data of our sample with descriptive data of historic controls, namely EORTC large scale cancer reference values (21) and representative German population values (11). Differences between our sample and historic samples were calculated using weighted means across men and women.
We also analyzed age effects on QoL reporting. In accordance with the EORTC reference data manual (15), we applied the following age cut-offs: <50, 50–59, 60–69, >70 years.
Analyses were performed using the software packages SPSS Statistics 23.0 (IBM Corporation, Armonk, NY, USA) and SAS 9.4 (SAS Institute, Cary NC).
Results
Patient characteristics
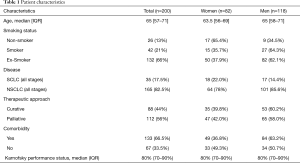
A total of 200 patients (82 female and 118 male) were enrolled (Table 1). Median age was 65 years (range, 39–91 years). Most patients had advanced disease (NSCLC stage IV n=77, 38.5%) and suffered from comorbidities (n=133, 66.5%). Non-small cell lung cancer was the predominant histological type (SCLC 17.5% vs. NSCLC 82.5%). Primary treatment at the time of questionnaire completion was either surgery (n=58), radio-chemotherapy (n=113) or targeted therapy (n=29).
Full table
Gender differences regarding functional and symptom scores
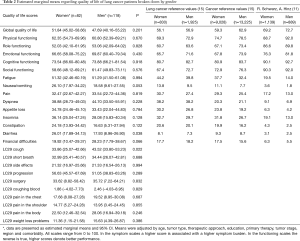
Table 2 presents the estimated marginal means (EMM) of QoL for women and men and the respective P values. Statistically significant differences were found only in two scales: cough severity was higher in men (EMM =43.52) than in women (EMM =33.86), P=0.022, and diarrhea was more pronounced in women (EMM 26.01) than in men (EMM 17.93), P=0.038. Other sizeable differences were observed with regard to nausea/vomiting (women EMM 26.10 and men EMM 18.58, P=0.053) and financial difficulties (women EMM 19.92 and men EMM 28.23, P=0.066), but the effects just fell short of the conventional level of statistical significance.
Full table
Gender differences and intensity of symptoms
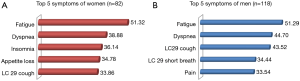
Figure 1 presents the five most prominent symptoms in descending order among women and men. Fatigue was the most pronounced symptom in women and men (EMM 51.32 and 51.29, respectively), followed by dyspnea (EMM 38.88 and EMM 44.70, respectively). Men, more so than women, reported higher symptom burden with regard to typical sensations related to lung cancer, such as dyspnea (EMM 44.70 vs. 38.88), coughing (EMM 43.52 vs. 33.86) and shortness of breath (EMM 34.44 vs. 32.99).
Comparisons with reference values
Table 2 depicts the original results of our study broken down by women and men, as well as historic QoL reference values (11,21).
The EORTC reference value manual contains QoL data from 9,028 female and 13,225 male patients pooled from numerous international studies with diverse cancer diagnoses (brain, breast, colorectal, gastric, genitourinary, gynecological, head and neck, leukemia, liver/bile/pancreas, lung, malignant lymphoma, malignant melanoma, mesothelioma, myeloma, non-Hodgkin lymphoma, esophageal, prostate, testicular) (15).
Table 2 reveals that our sample has higher symptom burden/worse functional QoL values than the EORTC lung cancer reference sample, particularly in the areas of diarrhea (mean difference between samples =13.70), nausea/vomiting (mean difference =10.87), social functioning (mean difference =−10.79), and physical functioning (mean difference =−10.38). Even more differences emerge with regard to the entire sample of cancer patients.
The highest differences were observed in comparison to the German reference data from a normal population. The most striking differences are found regarding role functioning (mean difference between samples =−35.37), fatigue (mean difference =34.21), dyspnea (mean difference =34.21), and social functioning (mean difference =−30.73).
All reported mean differences between samples considerably exceeded 10 score points, a commonly accepted criterion for a clinically meaningful difference (15).
Additional analyses
The gender differences observed with regard to diarrhea and coughing triggered additional subgroup analyses. We compared the use of targeted therapy (which would explain differences in diarrhea) between women and men. It turned out that women (18/29, 62.1%) received targeted therapy more frequently than men (11/29, 37.9%), chi2 =6.22, P=0.013, which corresponds with differences in diarrhea.
Furthermore, we calculated gender differences in smoking. Men are more likely to have a smoking history (either actual smokers or ex-smokers), with the proportion of non-smokers being lower (9/118, 7.6%) than in women (17/82, 20.7%) chi2 =6.22, P=0.025. Consequently, the number of packyears was higher in men (mean rank =96.76) than in women (mean rank =66.38), Mann-Whitney U =4.56, P<0.001. This corresponds with the higher self-reported coughing among men.
We also analyzed age effects on QoL reporting, since age is consistently and inversely (higher age, lower QoL) associated with QoL (11-17). There was not a single difference that approached statistical significance. A marginal and clinically plausible difference was obtained with regard to physical functioning with means being (P=0.069).
Discussion
Gender differences have been a subject of many analyses in QoL research (11-17). Based on a number of published large-scale studies we started with the notion that women would show lower levels of QoL (or, in reverse, higher symptom burden) than men.
In contrast to this hypothesis, we observed only one statistically significant difference to the disadvantage of women (diarrhea), whereas men reported significantly more coughing. This is at odds with other studies showing that women with lung cancer coughed significantly more than men (26,27). Subanalyses may help to explain these findings: women were more likely to receive targeted therapy, and targeted therapy, in turn, is associated with heightened levels of diarrhea (28). In our sample, most men were smokers and ex-smokers and had a higher number of packyears than women, which is a plausible precursor of coughing. However, coughing remains a symptom that is underresearched and poorly understood in the context of lung cancer. Other observational studies have failed to demonstrate an association with smoking (27). Further research is required in this area.
All other differences in functional and symptom scores were statistically not significant. Only one other study is in line with our results. In this study of 249 patients who had undergone lung surgery, no significant gender-associated difference in QoL was demonstrated (29).
Large scale population-based studies consistently reported gender differences. An investigation on the QoL of the general German population using the EORTC QLQ-C30 found that men reported consistently better functioning and less symptoms than women (11), and these findings were confirmed in a follow-up study (13). Population-based studies in Norway, Sweden and The Netherlands came to the same conclusion (14,16).
A Swedish population study on elderly cancer patients found that women reported more loneliness and fear than men (30). These population-based findings are echoed by an American NSCLC patient study, showing that men reported better QoL 12 weeks later after receiving early palliative care than women (31).
When it comes to the most common symptoms of lung cancer patients, earlier studies suggested tumor site-specific symptoms such as dyspnea, coughing, hemoptysis and pain (4,32). The present analysis portrayed a different picture; irrespective of gender, the number one symptom was fatigue. This is an important finding, since fatigue is increasingly being recognized as an unmet need of cancer patients (33). Cancer-related fatigue can occur at any time, at diagnosis, during treatment or even after treatment and is not only a burden on its own but is also associated with an increase in mortality (34,35).
A particularly striking finding is the difference in QoL scores of our sample of patients and reference data. Our patients report much worse QoL scores than the EORTC reference sample of lung cancer patients and even worse than a reference sample representing various cancer diagnoses. Even more pronounced are differences between our sample and data obtained in a German norm data survey. This pattern of result might help to explain the lack of gender differences: the reduced variation of QoL scores in our sample made the detection of group differences between women and men less likely.
The present analysis has several limitations. First, the study was not designed to detect gender differences; it was originally conducted to revise the EORTC questionnaire for QoL of lung cancer patients. Second, experience suggests that patients with advanced stages are less likely to complete questionnaires. Therefore, a specific gender difference study would require well-defined inclusion/exclusion criteria, allowing for the recruitment of patients across a wide range of disease and performance states, as well as an appropriate power calculation.
Conclusions
The present study adds to the literature in showing that the typical gender difference effect on QoL (women doing worse than men) may not be a universal phenomenon. Future studies have to show whether this lack in gender differences can be replicated, and whether it is due to considerable impairments in QoL, as may have been the case in our sample of patients with lung cancer undergoing treatment.
Acknowledgments
Parts of the data were presented at the congress of the German Respiratory Society, Dresden, March 2018 and at the congress of the European Respiratory Society, Paris, September 2018.
Funding: The conduct of this study was supported by a grant of the EORTC (Koller Lung 03/2010).
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1054
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1054
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1054). MK reports grants from EORTC during the conduct of the study as well as personal fees from Janssen-Cilag, Lilly, and MSD outside the submitted work. GI reports grant funding from the EORTC Quality of Life Group for the conduct of the study. CP serves as an unpaid editorial board member of Journal of Thoracic Disease from Sep 2018 to Aug 2020. The other authors have no conflicts to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethical Committee of the University of Regensburg, Germany (reference number 11-101-0024) and by the local ethical committees of the participating centers if required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients gave their informed consent prior to being enrolled into this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ulas A, Tokluoglu S, Kos M, et al. Lung cancer in women, a different disease: Survival differences by sex in Turkey. Asian Pac J Cancer Prev 2015;16:815-22. [Crossref] [PubMed]
- Kim H-R, Kim SY, Kim CH, et al. Sex-specific incidence of EGFR mutation and its association with age and obesity in lung adenocarcinomas: A retrospective analysis. J Cancer Res Clin Oncol 2017;143:2283-90. [Crossref] [PubMed]
- Rauma V, Salo J, Sintonen H, et al. Patient features predicting long-term survival and health-related quality of life after radical surgery for non-small cell lung cancer. Thorac Cancer 2016;7:333-9. [Crossref] [PubMed]
- Larsson M, Ljung L, Johansson BBK. Health-related quality of life in advanced non-small cell lung cancer: Correlates and comparisons to normative data. Eur J Cancer Care (Engl) 2012;21:642-9. [Crossref] [PubMed]
- Pinto JA, Vallejos CS, Raez LE, et al. Gender and outcomes in non-small cell lung cancer: An old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 2018;3:e000344. [Crossref] [PubMed]
- Jemal A, Miller KD, Ma J, et al. Higher Lung Cancer Incidence in Young Women Than Young Men in the United States. N Engl J Med 2018;378:1999-2009. [Crossref] [PubMed]
- Sakurai H, Asamura H, Goya T, et al. Survival differences by gender for resected non-small cell lung cancer: A retrospective analysis of 12,509 cases in a Japanese Lung Cancer Registry study. J Thorac Oncol 2010;5:1594-601. [Crossref] [PubMed]
- Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122. [Crossref] [PubMed]
- Ferrari R, Tanni SE, Lucheta PA, et al. Gender differences in predictors of health status in patients with COPD. J Bras Pneumol 2010;36:37-43. [Crossref] [PubMed]
- Pignataro FS, Bonini M, Forgione A, et al. Asthma and gender: The female lung. Pharmacol Res 2017;119:384-90. [Crossref] [PubMed]
- Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer 2001;37:1345-51. [Crossref] [PubMed]
- Hinz A, Singer S, Brähler E. European reference values for the quality of life questionnaire EORTC QLQ-C30: Results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol 2014;53:958-65. [Crossref] [PubMed]
- Waldmann A, Schubert D, Katalinic A. Normative data of the EORTC QLQ-C30 for the German population: A population-based survey. PLoS One 2013;8:e74149. [Crossref] [PubMed]
- Hjermstad MJ, Fayers PM, Bjordal K, et al. Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: The QLQ=C30 (+ 3). J Clin Oncol 1998;16:1188-96. [Crossref] [PubMed]
- Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 reference values; 2008.
- Derogar M, van der Schaaf M, Lagergren P. Reference values for the EORTC QLQ-C30 quality of life questionnaire in a random sample of the Swedish population. Acta Oncol 2012;51:10-6. [Crossref] [PubMed]
- van de Poll-Franse LV, Mols F, Gundy CM, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer 2011;47:667-75. [Crossref] [PubMed]
- Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs 2012;16:264-9. [Crossref] [PubMed]
- Banik A, Schwarzer R, Pawlowska I, et al. Women with family cancer history are at risk for poorer physical quality of life and lower self-efficacy: A longitudinal study among men and women with non-small cell lung cancer. Health Qual Life Outcomes 2017;15:62. [Crossref] [PubMed]
- Koller M, Warncke S, Hjermstad MJ, et al. Use of the lung cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical trials: A systematic review of the literature 20 years after its development. Cancer 2015;121:4300-23. [Crossref] [PubMed]
- Wang B, Hao N, Zhang X. Factors influencing the psychology and quality of life in lung cancer patients. Saudi Med J 2017;38:948-51. [Crossref] [PubMed]
- Koller M, Hjermstad MJ, Tomaszewski KA, et al. An international study to revise the EORTC questionnaire for assessing quality of life in lung cancer patients. Ann Oncol 2017;28:2874-81. [Crossref] [PubMed]
- Grimshaw J. SURGE (The SUrvey Reporting GuidelinE). In: Moher D, Altman DG, Wager E, Simera I, Schulz KF, editors. Guidelines for reporting health research: A user's manual. Chichester, West Sussex, Hoboken, NJ: John Wiley & Sons Ltd; 2014; 206-2013.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Fayers PM, Aaronson NK, Bjordal K, et al. EORTC QLQ-C30 scoring manual. 3rd; 2001.
- Kelsall A, Decalmer S, McGuinness K, et al. Sex differences and predictors of objective cough frequency in chronic cough. Thorax 2009;64:393-8. [Crossref] [PubMed]
- Harle ASM, Blackhall FH, Molassiotis A, et al. Cough in Patients With Lung Cancer: A Longitudinal Observational Study of Characterization and Clinical Associations. Chest 2019;155:103-13. [Crossref] [PubMed]
- Kroschinsky F, Stölzel F, Bonin S, von , et al. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit Care 2017;21:89. [Crossref] [PubMed]
- Sartipy U. Influence of gender on quality of life after lung surgery. Eur J Cardiothorac Surg 2010;37:802-6. [Crossref] [PubMed]
- Thomé B, Hallberg IR. Quality of life in older people with cancer -- a gender perspective. Eur J Cancer Care (Engl) 2004;13:454-63. [Crossref] [PubMed]
- Nipp RD, Greer JA, El-Jawahri A, et al. Age and Gender Moderate the Impact of Early Palliative Care in Metastatic Non-Small Cell Lung Cancer. Oncologist 2016;21:119-26. [Crossref] [PubMed]
- Latimer KM, Mott TF. Lung cancer: Diagnosis, treatment principles, and screening. Am Fam Physician 2015;91:250-6. [PubMed]
- Madsen UR, Groenvold M, Petersen MA, et al. Comparing three different approaches to the measurement of needs concerning fatigue in patients with advanced cancer. Qual Life Res 2015;24:2231-8. [Crossref] [PubMed]
- Horneber M, Fischer I, Dimeo F, et al. Cancer-related fatigue: Epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int 2012;109:161-71. [PubMed]
- Fischer I, Riedner C, Bojko P, et al. Consultation Program for Patients with Cancer-Related Fatigue: A Systematic Evaluation of the Experiences of the Bavarian Cancer Society. Oncol Res Treat 2016;39:646-51. [Crossref] [PubMed]


