
Changes in children’s respiratory morbidity and residential exposure factors over 25 years in Chongqing, China
Introduction
Childhood respiratory morbidity and mortality contribute significantly to global burden of disease. Based on the Global Burden of Disease assessments, lower respiratory infections have always ranked among the top 10 of leading causes of death in children and adolescents (1,2). The prevalence of children’s respiratory diseases is increasing in the world, particularly in developing countries (3,4). In general, children are more vulnerable to adverse exposures compared with adults, since some of the organ systems including immune and respiratory system are still in development during childhood (5-7). Spending more than 16 hours per day indoors on average, children are considered to be more vulnerable to residential environmental risk factors (8,9). Hence, the impact of residential exposures on children’s respiratory health is of important concerns.
Previous studies have showed that indoor exposures were associated with respiratory morbidity. Two review articles concluded that environmental tobacco smoke exposure was a recognized major influence on the risk of both acute and chronic respiratory illness, associated with respiratory tract infection, wheezing and asthma in young children (4,10). In addition to tobacco exposure, the use of unclean fuels has also been reported as a contributing factor to the prevalence of respiratory disease in children. Indoor cooking with unclean fuels including biomass, kerosene, wood and charcoal was associated with a higher incidence of respiratory symptoms including wheezing (11,12). Studies have also reported that inhaling cooking oil fumes was associated with lung function reductions and respiratory symptoms in children (13,14). Since studies relating to fuels use did not control cooking smoke inhalation, and studies of smoke inhalation did not control fuels use, making their relationship with children’s respiratory health unclear.
In 1990s, the China-US Science and Technology Cooperation Project conducted a survey in four Chinese cities including Chongqing, Guangzhou, Lanzhou, and Wuhan, which aimed to study the relationship between air pollution and respiratory health in children (the 1993 study) (15). The study showed that environmental tobacco smoke and household coal use for cooking and heating were risk factors of children’s respiratory health (16,17). Chongqing, located in southwest China, one of the largest industrial, transportation and financial center city, was selected as one of point city in the study due to its high-density population and high prevalence of sulfur-rich household coal use in the 1993 study (with the highest ambient concentration of sulfur dioxide among the four cities) (18).
Chongqing has experienced a rapid economic development and urbanization since the 1993 study. Since 2000, a series of measures for environment improvement such as “the clean energy project” and “the blue sky action” have been implemented in Chongqing. All these development and measures resulted in a profound change in household characteristics (e.g., fuels for cooking and heating in winter, kitchen ventilation, cooking habit, parental smoking etc.). For example, with the universal use of clean energy such as natural gas instead of burning coal for cooking and electric air conditioning device instead of coal for heating, the indoor air pollution exposures must have improved substantially.
Chongqing’s GDP grew 31 times from 60.853 billion in 1993 to 1.9425 trillion RMB in 2017 (19). It is conceivable that lifestyle and household characteristics in Chongqing have also changed dramatically over the past 25 years. Therefore, it is important to re-evaluate the prevalence of respiratory diseases and relevant risk factors in children. We hypothesize that the economic development in Chongqing over the 25-year period has resulted in changes in residential environmental exposures, children’s respiratory morbidity, and the associations between residential exposure factors and respiratory outcomes. This study aims to evaluate the changes in residential exposures, the prevalence of respiratory symptoms and diseases in school-age children as well as their associations.
Methods
Study population
In the 1993 study, two districts were selected with one being an inner city district of relatively higher ambient air pollution level and the other being a suburban district of lower air pollution level. In each district, one primary school was chosen, and 1,452 children in grades 2–6 and their parents accepted our questionnaire survey in the 1993. In the 2017 study, we selected a school close to the original school in the inner city district and the suburban district respectively; and we surveyed 2,126 families of children in grades 1–6.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Ethics Committee of Chongqing Medical University (approval number 2017005) as well as the IRB of Duke Kunshan University (approval number FWA00021580). Written informed consent was obtained from all parents/guardians.
Questionnaire survey
The questionnaire for 1993 study was adapted from the American Thoracic Society Epidemiologic Standardization Project questionnaire (ATS-DLD-78-C) (20,21). In the 2017 study, we kept the questionnaire content the same as much as possible with additions and modifications made to reflect some unique changes between 1993 and 2017. In summary, through the survey, we collected information on residential history, lifestyle, household characteristics, and children’s and parents’ health histories, fuels for home cooking and heating, kitchen ventilation devices, parental smoking status, parental respiratory health status, and children’s sleep condition. The collection of questionnaire data in 1993 study has been previously described (7,10). During the 2017 survey, requirements, and precautions for answering questionnaire were explained in detail to all participating children and their parents or guardians. Rigorous quality review on the returned questionnaires was conducted by same investigators. Unqualified questionnaires (i.e., those with too many missing answers and unclear answers) were returned to the participants for refilling. If a questionnaire had some answers missing, the study staff filled the missing information via a follow-up telephone call.
Children’s respiratory morbidity outcomes were determined from the multiple health outcomes collected in the questionnaires and were defined as follows. (I) Cough: a ‘yes’ answer to any of the following questions: ‘Has this child ever coughed when he/she has a cold?’ or ‘Has this child ever coughed without colds?’. (II) Phlegm: a ‘yes’ answer to any of the following questions: ‘Does this child have phlegm from the chest when he/she has colds?’ or ‘When this child does not have a cold, does he/she have phlegm from the chest?’. (III) Wheezing: a ‘yes’ answer to any of the following questions: ‘Has this child ever wheezed when he has colds?’ or ‘Has this child ever wheezed when he/she does not have colds?’ or ‘Has this child ever had wheezing on most days or nights?’. (VI) Asthma: a ‘yes’ answer to the question ‘Has a doctor ever diagnosed asthma in this child?’. (V) Bronchitis: a ‘yes’ answer to the question ‘Has a doctor ever diagnosed bronchitis in this child?’.
Statistical methods
We used t tests and chi-square tests to compare the residential exposure factors, demographics, and parental respiratory diseases between the two studies. We calculated odds ratios to compare the prevalence of children’s respiratory symptoms and diseases between the two periods, adjusting for covariates for residential, demographics, and parental respiratory diseases. Unconditional logistic regression models were used to analyze the relationship between residential exposures and children’s respiratory diseases and symptoms in each of the two studies. Important covariates, including age, sex, parental education level, parental asthma, and parental bronchitis, were adjusted in logistic regression models. Statistical significance was achieved when P<0.05. SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) was used to conduct data analysis.
Results
Comparison in demographics, residential factors, and parental respiratory diseases between the two study times
Residential factors, demographics, and parental respiratory diseases of two studies were presented in Table 1. Compared to those of the 1993 study, most of the indoor exposure risk factors that were considered as indicators of residential environment have improved in the 2017 study, such as sleep in shared room (χ2 14.2, P<0.001), cooking with coal (χ2 58.5, P<0.001), poor kitchen ventilation (χ2 197.7, P<0.001), paternal (χ2 275.5, P<0.001) and maternal smoking (χ2 4.8, P=0.029), whereas cooking frequency per week increased (χ2 65.1, P<0.001). For demographics, children’s age was older (9.39±1.60 vs. 8.73±1.16, P<0.001), while maternal education level was higher in the 2017 study (χ2 65.7, P<0.001). Parental asthma and bronchitis prevalence rates were lower among participants in the 2017 study (paternal asthma: χ2 12.8, P<0.001; maternal asthma: χ2 8.7, P=0.003; paternal bronchitis: χ2 36.0, P<0.001; maternal bronchitis: χ2 19.5, P<0.001).
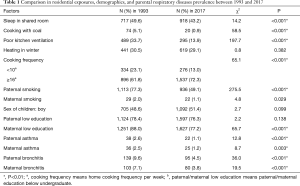
Full table
Change in children’s respiratory morbidity
Adjusted ORs for children’s respiratory morbidity in 2017 in reference to 1993 were shown in Table 2. The prevalence of children’s wheezing in 2017 (5.8%) was significantly lower than that in 1993 (16.5%) (OR: 0.377, 95% CI: 0.288, 0.494). However, the prevalence of children’s bronchitis in 2017 (23.6%) was significantly higher than that in 1993 (17.7%) (OR: 1.888, 95% CI: 1.540, 2.314). No statistically significant difference was found in the prevalence of children’s cough, phlegm, and asthma between the two studies.

Full table
Associations between children’s respiratory morbidity and residential exposures, demographics, and parental respiratory diseases
Residential exposures
Multivariable logistic regression analysis was conducted to test association between children’s respiratory morbidity and residential exposures as well as parental factors (education level, having asthma or bronchitis) in 1993 and 2017, respectively. Results are shown in Table 3. The adjusted analysis of the 1993 data found that poor kitchen ventilation and parental smoking were associated with increased risks for children’s wheezing (OR: 1.39, 95% CI: 1.02, 1.90) and for bronchitis (OR: 1.51, 95% CI: 1.02, 2.21), respectively, while heating in winter was significantly associated with an increased risk of phlegm (OR: 1.40, 95% CI: 1.03, 1.90) and an increased risk for wheezing (OR: 1.47, 95% CI: 1.07, 2.01). However, these residential exposure factors were no longer associated with the children’s respiratory diseases in the 2017 study.
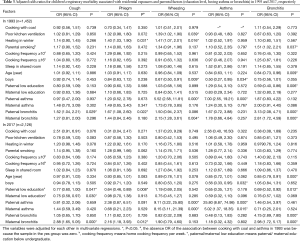
Full table
Demographics
Demographic indicators included children’s age, sex, and parental education. Older age in children was associated with a lower risk of bronchitis in both studies (OR: 0.84, 95% CI: 0.63, 1.11 in the 1993 study and OR: 0.85, 95% CI: 0.72, 1.01 in the 2017 study). Boys had a lower risk of wheezing in 1993 (OR: 0.67, 95% CI: 0.50, 0.91) and asthma in 1993 (OR: 0.50, 95% CI: 0.27, 0.95) and in 2017 (OR: 0.56, 95% CI: 0.33, 0.95) than girls. The children whose father had lower education attainment (below undergraduate) had lower risk of bronchitis (OR: 0.59, 95% CI: 0.40, 0.86) in the 1993 study and a lower risk of cough (OR: 0.77, 95% CI: 0.60, 1.00), phlegm (OR: 0.64, 95% CI: 0.46, 0.89), and bronchitis (OR: 0.69, 95% CI: 0.52, 0.92) in the 2017 study. Children whose mothers had lower education attainment had a lower risk of cough only in the 2017 study (OR: 0.75, 95% CI: 0.58, 0.97).
Parental respiratory diseases
Paternal asthma was significantly associated with children’s wheezing in both 1993 (OR: 5.52, 95% CI: 2.58, 11.81) and 2017 (OR: 9.11, 95% CI: 3.22, 25.80). Paternal asthma was also significantly associated with children’s asthma in both 1993 (OR: 7.00, 95% CI: 2.55, 19.21) and in 2017 (OR: 25.80, 95% CI: 8.87, 74.98). However, maternal asthma was significantly associated with children’s wheezing (OR: 8.15, 95% CI: 3.11, 21.39) and asthma (OR: 5.02, 95% CI: 1.37, 18.35) only in the 2017 survey. Paternal bronchitis was associated with children’s cough (OR: 1.52, 95% CI: 1.04, 2.21), phlegm (OR: 1.67, 95% CI: 1.08, 2.60), and bronchitis (OR: 3.13, 95% CI: 2.08, 4.71) in 1993, and only associated with bronchitis (OR: 4.75, 95% CI: 2.89, 7.80) in 2017. Maternal bronchitis was associated with children’s wheezing (OR: 2.15, 95% CI: 1.28, 3.61) and bronchitis (OR: 2.81, 95% CI: 1.72, 4.59) in 1993, and was associated with cough (OR: 2.88, 95% CI: 1.65, 5.05), phlegm (OR: 2.16, 95% CI: 1.18, 3.93) and bronchitis (OR: 2.98, 95% CI: 1.72, 5.17) in 2017.
Discussion
This study compared the residential exposures, the prevalence of respiratory symptoms and diseases adjusted for covariates, and their associations among school-age children in Chongqing between 1993 and 2017.
This research found that most residential exposure factors, except home cooking frequency, had been improved in 2017 compared to those in 1993. High cooking frequency was considered as a risk factor due to its relation with cooking fumes and non-clean fuels (e.g., coal), which had been reported to be a risk factor of respiratory disease in children. However, with the popularity of natural gas, range hood and exhaust fans, the negative impact of high cooking frequency on children’s respiratory system was significantly weakened. Therefore, the residential potential exposures included in this study showed an overall trend of improvement, which was likely to be beneficial to the respiratory health of children.
Consistent with our hypothesis, the prevalence of wheezing in 2017 (5.8%) was significantly lower than that in 1993 (16.5%) and similar to the prevalence (5.2%) reported in 2015 previously for Chongqing school children (22). After controlling for the covariates, the prevalence of wheezing remained lower than that in 1993 study (OR: 0.377, 95% CI: 0.288, 0.494). In contrast, prevalence of children’s bronchitis in 2017 (23.6%) rose compared to study in 1993 (17.7%), and the result remained the same after controlling for covariates (OR: 1.888, 95% CI: 1.540, 2.314). In the context of the overall improvement of residential environment, the rising prevalence of bronchitis suggests that factors other than residential exposures may have played a larger role in the development bronchitis. Studies found an increased risk for bronchitis associated with traffic-related air pollution (23,24), which was in line with the rapid increase of vehicle population in Chongqing in recent years. Studies also reported viral or bacterial infection, being obese, cold weather, air quality had influence on bronchitis (25-28), which were not taken into account in this analysis. We note that the lack of data on many other factors is a major limitation of our study. Similarly, the prevalence of bronchitis among children in Chongqing was 24.40% in 2013 as reported in a previous study using the same evaluation method (29). However, we could not find data showing long-term temporal trend in bronchitis prevalence in Chongqing or in China.
In the multivariate analyses of the 1993 data, poor kitchen ventilation was associated with higher prevalence of children’s wheezing after controlling for cooking with coal, which suggests that cooking fumes may have contributed to children’s wheezing. Also, heating in winter was a risk factor for phlegm and wheezing. A study has found that heating in winter can worsen indoor air quality (30), leading to an increased risk for respiratory symptoms. Children exposed to parental smoking increased their risk for bronchitis by 1.5 times in 1993. However, these factors were no longer associated with children’s respiratory symptoms and diseases in 2017, suggesting that the improvement of residential factors weakened its effect on respiratory diseases. A review of 52 studies also found that improving home environments helped reduce respiratory problems such as asthma and chronic bronchitis (31).
We found that both paternal asthma and maternal asthma significantly increased children’s risks for wheezing and asthma. This finding is consistent with previous findings that parental asthma increased children’s asthma risk in Chongqing (32) and elsewhere (33-35). This child-parent association may be due to the genetic heredity and/or due to the fact that children and their parents are exposed to many common environmental factors. Future studies are recommended to examine the reasons for this association.
This study has several notable limitations. Firstly, the relationship between residential factors and health effects is a very complex issue. This study is only a cross-sectional comparison of two surveys conducted with a 25-year interval. The comparison may have missed other unmeasured time-varying factors (e.g., medical care, immunizations, pets, nutrition, and exercise) (36-39). Due to data constraints, we can only conduct a comparative analysis on the existing variables. Secondly, the data in prevalence of respiratory symptoms and diseases as well as residential exposure factors in this study were derived from self-report rather than measurements, which are subject to recall bias.
As the global health community continues to prioritize child and adolescent health during the Sustainable Development Goal era, careful attention should also be placed on identifying, detecting, and controlling related environmental risk factors for childhood respiratory health.
Conclusions
Our study found improvements of several residential exposure factors, a significant decline of prevalence for wheezing, and a significant increase of prevalence for bronchitis from 1993 to 2017 in school children of Chongqing. Poor kitchen ventilation, heating in winter and parental smoking as important risk factors for children’s respiratory symptoms and diseases in 1993. However, these factors were largely improved compared to the 1993 conditions and no longer associated with children’s respiratory morbidity in 2017.
Acknowledgments
We would like to thank the students and their parents for their participation. We are grateful to the leaders and teachers of the two primary schools for their kind assistance with recruitment and data collection. We would like to thank Dr. Laurent Irakoze from First Affiliated Hospital of Chongqing Medical University for edits on the paper.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Junfeng Jim Zhang, Howard Kipen and Haidong Kan) for the series “Children’s Respiratory Health and Air Quality” published in Journal of Thoracic Disease. The article was sent for peer review organized by the Guest Editors and the editorial office.
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-19-crh-aq-005
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-crh-aq-005). The series “Children’s Respiratory Health and Air Quality” was commissioned by the editorial office without any funding or sponsorship. JJZ served as the unpaid Guest Editor of the series. JJZ also serves as an unpaid editorial board member of Journal of Thoracic Disease. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Chongqing Medical University Ethics Committee (No. 2017005) and Duke Kunshan University IRB (No. FWA00021580), respectively. Informed consent was obtained from both participating children and their parents or guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Child and Adolescent Health Collaboration, Kassebaum N, Kyu HH, et al. Child and Adolescent Health From 1990 to 2015: Findings From the Global Burden of Diseases, Injuries, and Risk Factors 2015 Study. JAMA Pediatr 2017;171:573-92. [Crossref] [PubMed]
- Vanker A, Gie RP, Zar HJ. The association between environmental tobacco smoke exposure and childhood respiratory disease: a review. Expert Rev Respir Med 2017;11:661-73. [Crossref] [PubMed]
- Campbell-Lendrum D, Pruss-Ustun A. Climate change, air pollution and noncommunicable diseases. Bull World Health Organ 2019;97:160-1. [Crossref] [PubMed]
- Tong S. Air pollution and disease burden. The Lancet. Planetary health 2019;3:e49-50. [Crossref] [PubMed]
- Lin WW, Chen ZX, Kong ML, et al. Air Pollution and Children's Health in Chinese. Adv Exp Med Biol 2017;1017:153-80. [Crossref] [PubMed]
- Yang CY, Chiu JF, Cheng MF, et al. Effects of indoor environmental factors on respiratory health of children in a subtropical climate. Environ Res 1997;75:49-55. [Crossref] [PubMed]
- Azad SMY, Bahauddin KM, Uddin H, et al. Indoor air-pollution and prevalence of acute respiratory infection among children in rural area of Bangladesh. Clarion 2014;3:7-20.
- Lu Y, Lin S, Lawrence WR, et al. Evidence from SINPHONIE project: Impact of home environmental exposures on respiratory health among school-age children in Romania. Sci Total Environ 2018;621:75-84. [Crossref] [PubMed]
- Tsai CH, Huang JH, Hwang BF, et al. Household environmental tobacco smoke and risks of asthma, wheeze and bronchitic symptoms among children in Taiwan. Respir Res 2010;11:11. [Crossref] [PubMed]
- Jones LL, Hashim A, McKeever T, et al. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res 2011;12:5. [Crossref] [PubMed]
- Nandasena S, Wickremasinghe AR, Sathiakumar N. Respiratory health status of children from two different air pollution exposure settings of Sri Lanka: A cross-sectional study. Am J Ind Med 2012;55:1137-45. [Crossref] [PubMed]
- Owusu Boadi K, Kuitunen M. Factors affecting the choice of cooking fuel, cooking place and respiratory health in the Accra metropolitan area, Ghana. J Biosoc Sci 2006;38:403-12. [Crossref] [PubMed]
- Nenna R, Cutrera R, Frassanito A, et al. Modifiable risk factors associated with bronchiolitis. Ther Adv Respir Dis 2017;11:393-401. [Crossref] [PubMed]
- Qian Z, He Q, Kong L, et al. Respiratory responses to diverse indoor combustion air pollution sources. Indoor Air 2007;17:135-42. [Crossref] [PubMed]
- Qian Z, Zhang J, Wei F, et al. Long-term ambient air pollution levels in four Chinese cities: inter-city and intra-city concentration gradients for epidemiological studies. J Expo Anal Environ Epidemiol 2001;11:341-51. [Crossref] [PubMed]
- Zhang J, Qian Z, Kong L, et al. Effects of Air Pollution on Respiratory Health of Adults in Three Chinese Cities. Arch Environ Health 1999;54:373-81. [Crossref] [PubMed]
- Qian Z. Factor analysis of household factors: are they associated with respiratory conditions in Chinese children? Int J Epidemiol 2004;33:582-8. [Crossref] [PubMed]
- Yin Z, Huang X, He L, et al. Trends in ambient air pollution levels and PM2.5 chemical compositions in four Chinese cities from 1995 to 2017. J Thorac Dis 2020;12:6396-410.
- National Bureau of Statistics. China. 1993-2017. Available online: http://www.stats.gov.cn/
- Ferris BG Jr. Epidemiology standardization project (American Thoracic Society). Am Rev Resp Dis 1978;118:1-120. [PubMed]
- The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhino conjunctivitis, and atopic eczema: ISAAC. Lancet 1998;351:1225-32. [Crossref]
- Fan MY, Tang X, Huang W, et al. Effect of air pollution on respiratory health in school-aged children in the main urban area of Chongqing, China. Zhongguo Dang Dai Er Ke Za Zhi 2017;19:436-40. [PubMed]
- Bai L, Su X, Zhao D, et al. Exposure to traffic-related air pollution and acute bronchitis in children: season and age as modifiers. J Epidemiol Community Health 2018;72:426-33. [Crossref] [PubMed]
- Pino P, Walter T, Oyarzun M, Villegas R, Romieu I. Fine particulate matter and wheezing illnesses in the first year of life. Epidemiology 2004;15:702-8. [Crossref] [PubMed]
- Mejza F, Gnatiuc L, Buist AS, et al. Prevalence and burden of chronic bronchitis symptoms: results from the BOLD study. Eur Respir J 2017;50:1700621. [Crossref] [PubMed]
- Kinkade S, Long NA. Acute Bronchitis. Am Fam Physician 2016;94:560-5. [PubMed]
- Lee YL, Chen YC, Chen YA. Obesity and the occurrence of bronchitis in adolescents. Obesity 2013;21:E149-53. [Crossref] [PubMed]
- Berhane K, Chang CC, McConnell R, et al. Association of Changes in Air Quality With Bronchitic Symptoms in Children in California, 1993-2012. JAMA 2016;315:1491-501. [Crossref] [PubMed]
- Tang JY. Study on air particulate pollution and its effect on children's respiratory system in Chongqing. Chongqing Medical University,2016 (in Chinese).
- Xiao Q, Ma Z, Li S, et al. The impact of winter heating on air pollution in China. PLoS One 2015;10:e0117311. [Crossref] [PubMed]
- Le Cann P, Paulus H, Glorennec P, et al. Home Environmental Interventions for the Prevention or Control of Allergic and Respiratory Diseases: What Really Works. J Allergy Clin Immunol Pract 2017;5:66-79. [Crossref] [PubMed]
- Yang H. Survey on the prevalence rate and risk factors of childhood asthma aged 0 to 14 in Chongqing city. Chongqing: Chongqing Medical University, 2012.
- Joseph M, Zoubeidi T, Al-Dhaheri SM, et al. Paternal asthma is a predictor for childhood asthma in the consanguineous families from the United Arab Emirates. J Asthma 2009;46:175-8. [Crossref] [PubMed]
- Abramson M, Kutin JJ, Raven J, et al. Risk factors for asthma among young adults in Melbourne, Australia. Respirology 1996;1:291-7. [Crossref] [PubMed]
- Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers vs. fathers: a meta-analysis. PLoS One 2010;5:e10134. [Crossref] [PubMed]
- Gilliland FD, Berhane KT, Li YF, et al. Children's lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am J Epidemiol 2003;158:576-84. [Crossref] [PubMed]
- Romieu I, Barraza-Villarreal A, Escamilla-Núñez C, et al. Dietary intake, lung function and airway inflammation in Mexico City school children exposed to air pollutants. Respir Res 2009;10:122. [Crossref] [PubMed]
- Berntsen S, Wisløff T, Nafstad P, et al. Lung function increases with increasing level of physical activity in school children. Pediatr Exerc Sci 2008;20:402-10. [Crossref] [PubMed]
- Ji J, Wang SQ, Liu YJ, et al. Physical Activity and Lung Function Growth in a Cohort of Chinese School Children: A Prospective Study. PLoS One 2013;8:e66098. [Crossref] [PubMed]


