Prognostic role of p53 and Ki-67 immunohistochemical expression in patients with surgically resected lung adenocarcinoma: a retrospective study
Introduction
Lung cancer is the leading cause of cancer deaths worldwide, contributing to approximately 1.6 million deaths each year despite advances in diagnosis and treatment; its incidence rate is steadily increasing in industrialized countries (1). In a recent survey during the year 2011 in Korea, the age-standardized incidence rates for lung cancer per 100,000 people were 46.0% and 15.1% for men and women, respectively. The most common site of cancer death in both sexes was also lung [for men, crude rate (CR), 45.9%; age-standardized rate (ASR), 35.0%; for women, CR, 17.4%; ASR, 9.1%], and the 5-year lung cancer survival rate from 2007 to 2011 was 18.3%, and 26.8%, for men and women, respectively (2).
Approximately 80% of lung cancers are classified histologically as non-small cell lung cancers (NSCLC), of which adenocarcinoma is the most common type (3). Recently, strategies for lung cancer treatment have focused on inhibiting targeted molecules or oncogenic pathways. Such examples are receptor tyrosine kinases, which have already provided us with new and preferred therapeutic options (4,5). Innovative approaches are being used to develop biomarkers of lung cancer risk and prognosis. Validation studies, however, should be underway for future introduction in clinical practice. Several clinical and pathologic variables are useful for assessing the prognosis of lung cancer patients.
Mutations of p53 gene are usually described as abnormal DNA sequences in p53, and are the most common molecular alteration in human cancer (6). Ki-67 antigen is one of several cell-cycle regulating proteins and is associated with ribosomal RNA transcription (7). Immunohistochemical (IHC) expression of p53 and Ki-67 is usually interpreted as likely indicating a p53 gene mutation and a nuclear marker for cell proliferation (8,9). Accordingly, their detection in the tumor tissue has taken on considerable importance in prognosis and treatment in lung cancer. While nucleotide sequencing is the most reliable technique to detect gene mutation, it is time consuming, laborious, complicated, and costly. Conversely, an IHC analysis can rapidly detect the altered protein produced by the mutant gene, although it is neither 100% specific nor 100% sensitive (10).
We conducted a retrospective analysis to determine the prognostic significance of IHC expression of p53 and Ki-67 in tumor samples from patients with surgically resected lung adenocarcinoma at Hallym University Medical Center in Korea.
Materials and methods
Patients and lung cancer specimens
All patients were initially diagnosed with NSCLC at the Hallym University-affiliated hospitals, including the Dongtan, Hangang, Chuncheon, Kangnam, Kangdong, and Hallym University Sacred Heart Hospital, between 2002 and 2013. A total of 323 patients received surgical treatment. Of these, primary lung adenocarcinomas were identified in 136 patients. All smokers had a ≥10 pack-year history of smoking. Patient follow-up information was obtained through review of hospital records or direct patient contact.
All specimens were obtained from resected tumors. The hematoxylin and eosin (H&E) slides from each patient were reviewed and histologically classified according to the revised TNM classification of lung cancer (11). Clinical and pathologic information was also obtained, including the type of operation, age, sex, co-morbidities, smoking history, tumor differentiation, regional lymph node involvement, pathologic stage, absolute lymphocyte count (ALC), follow-up status, tumor recurrence and progression, and survival. Lymphopenia was defined as an ALC of <1,000/μL at the diagnosis of lung cancer. Two patients were lost to follow-up before completion of the study (Figure 1).
IHC staining
For IHC staining, 4-micrometer-thick sections were deparaffinized. IHC staining was performed using the Ventana BenchMark XT immunostainer (Ventana Medical Systems, Germany) automated slide preparation system; the primary antibodies were a mouse monoclonal anti-human Ki-67 antibody (clone MIB1, DAKO, Denmark, IS626, 1:100), and a mouse monoclonal p53 antibody (DO7, Cell Marque, California, USA, 453M-96, 1:100).
Simultaneous staining of a known p53 positive case and a Ki-67 positive case were used as the positive controls. Negative controls were obtained by applying phosphate-buffered saline instead of Ki-67 and p53 antibodies.
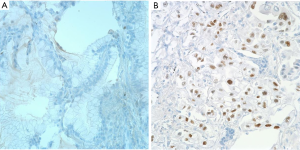
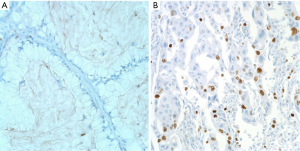
A p53 expression by IHC was graded as negative (<5% tumor cells) or positive (>5% tumor cells) (Figure 2A,B) (12). IHC expression level of Ki-67 was estimated as the percentage of positive tumor cells within one high-power field, and were initially divided into four grades (negative, <1%; low, 1-10%; moderate, 10-50%; high, >51%) (13,14). Next, we reclassified Ki-67 expression levels into two groups (low, <10%; high, ≥10%) (Figure 3A,B) (15-17). The results of IHC were judged independently by two pathologists (JW Shim and YH Choi), who were unware of the clinical data, and consensus was reached for any discordant cases. The Histoscore for IHC analysis was utilized as a reference standard (18).
Statistical analysis
Associations between p53 and Ki-67 and clinical and pathologic variables were analyzed by using the χ2 test. A logistic regression model was used to assess multiple associations. Survival time was determined as the time from tumor resection to death from any cause, and censored at the last observation date that each patient was known to be alive. The associations between individual clinical and pathologic variables, such as age, sex, peripheral ALC, pathologic stage, tumor differentiation, T stage, N stage, p53, Ki-67, and survival, were assessed by using the Cox proportional hazards regression model. Survival probabilities and poor outcome (tumor recurrence, progression, or death) were estimated by using the Kaplan-Meier method and compared with the log-rank test. An independent analysis was also performed for stages I, II and >III. A P value of <0.05 was considered significantly. Statistical analyses were performed using the dBSTAT software version 4.0 (dBSTAT Inc., Chuncheon, Korea).
Results
Patient characteristics
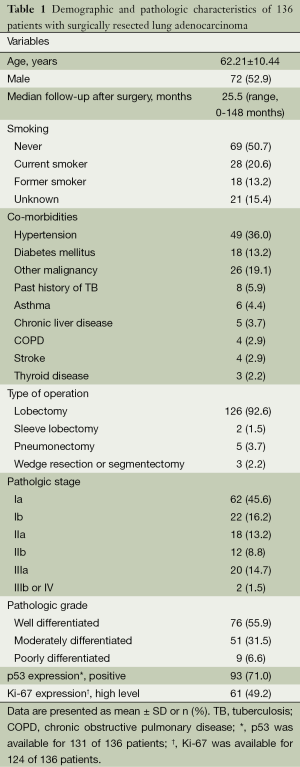
The patients with lung adenocarcinoma consisted of 72 men and 64 women with ages ranging from 37 to 82 years (mean 62.2 years). The median follow-up after the operation was 25.5 months (range, 0-148 months). Regarding smoking history, 28 patients (20.6%) were current smokers, 69 (50.7%) were non-smokers, 18 (13.2%) were former smokers, and 21 patients (15.4%) had an unknown smoking history. Surgical operations included lobectomy in 126 patients (92.6%), pneumonectomy in 5 (3.7%), sleeve lobectomy in 2 (1.5%), and wedge resection or segmentectomy in 3 (2.2%). Pathologic stage Ia was observed in 62 patients (45.6%), Ib in 22 (16.2%), IIa in 18 (13.2%), IIb in 12 (8.8%), IIIa in 20 (14.7%), IIIb in 1 (0.7%), and IV in 1 (0.7%). IHC staining of p53 and Ki-67 was positive in 93 (71.0%) and showed high expression level in 61 (49.2%) cases, respectively (Table 1).
Full table
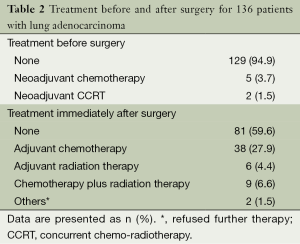
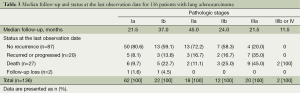
Among 136 patients with lung adenocarcinoma, 7 (5.2%) were treated with neoadjuvant therapy and 53 (39.0%) were treated with adjuvant therapy (Table 2). At the last observation date, 87 patients (64.0%) did not show recurrence of the tumors, however, 20 (14.7%) had either encountered recurrence or progression of the disease; 27 cases (19.9%) died during the follow-up period (Table 3).
Full table
Full table
Data are presented as n (%).
Associations between the clinical and pathologic variables, and IHC expression of p53 and Ki-67
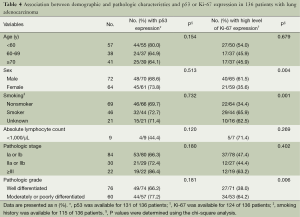
Associations between the clinical and pathologic variables, and p53 and Ki-67 expression by IHC were determined using the χ2 test. Whereas p53 was not associated with other variables, high level of Ki-67 expression was significantly associated with male sex, smoking history, and poorer differentiation of the tumor (P=004, P=0.001, and P=0.006, respectively) (Table 4). Of these variables, moderately or poorly differentiated tumor grade was independently associated with high expression level of Ki-67 [OR =3.091, 95% confidence interval (CI) =1.336, 7.147; P=0.008], compared to well-differentiated tumor grades (Table 5).
Full table

Full table
Prognostic value of p53 and Ki-67 IHC expression
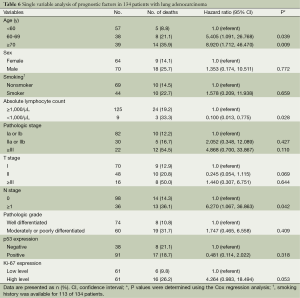
After excluding two patients who were lost to follow-up, a total of 134 patients with lung adenocarcinoma were included for analysis using the Cox proportional hazards regression model (Figure 1). The median follow-up was 26 months (range, 0-148 months) among all patients (n=134), and 35 months (range, 1-148 months) among survivors (n=107). Patients with age >60 years, or with regional lymph node metastasis were lower survival significantly (HR =5.405, 95% CI =1.091, 26.768; P=0.039; HR =6.270, 95% CI =1.067, 36.863; P=0.042, respectively), compared to those aged <60 years, or without lymph node metastasis (Table 6).
Full table
Associations between p53 and Ki-67 IHC expression and overall survival and poor outcome
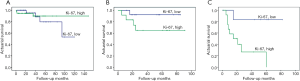
No significant associations were found between p53 and Ki-67 IHC expression (P>0.05). The influence of p53 and Ki-67 on actuarial survival or poor outcome was analyzed by using the Kaplan-Meier method. Between a combination of all pathologic stages (I, II, and ≥III), p53 showed no association with patient survival (log-rank test, χ2=0.148; P=0.701) (Figure 4); the same pattern was observed upon fitting the method separately to stage I, II or ≥III (Figure 5A-C). On the other hand, high level of Ki-67 expression correlated significantly with decreased survival (log-rank test, χ2=5.637; P=0.018) in stages combined (Figure 4); however, the effect was limited to stage ≥III (log-rank test, χ2=5.939; P=0.015), whereas such an effect was not observed separately among stage I or II (Figure 6A-C).
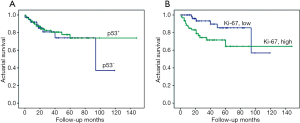

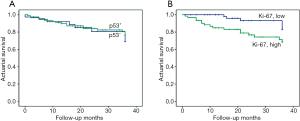
Overall, the 3-year cumulative survival rates were 90.6% in stage I, 80.7% in stage II, and 46.2% in stage ≥III, respectively (data not shown). The association between p53 and Ki-67 immuno-expression and 3-year overall survival was estimated for all stages. High expression level of Ki-67 was significantly associated with worse patient outcome (log-rank test, χ2=4.936; P=0.026); however, this correlation was absent for p53 (log-rank test, χ2=0.216; P=0.642) (Figure 7A,B).
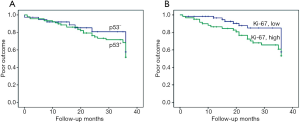
Of the 134 cases with follow-up, overall worse cumulative outcomes including tumor recurrence, progression, or death, were observed in 19 (23.2%) stage I patients (n=82), 10 (33.3%) stage II patients (n=30), and 18 (81.8%) stage ≥III patients (n=22) during the initial 3-year follow-up period. The associations between immuno-expression of p53 and Ki-67 and poor outcome were assessed for all patients with stages I, II and ≥III. However, no statistically significant difference was observed for both p53 and Ki-67 (log-rank test, χ2=0.439; P=0.508 and χ2=1.650; P=0.199) (Figure 8A,B).
Discussion
Approximately 40% of lung cancers are adenocarcinomas that occur mainly in current or former smokers. It is also the most common type of lung cancer observed in non-smokers (19). The incidence of lung cancer is steadily increasing in Korean women although the majority of them are non-smokers; histologically, the tumors are predominantly adenocarcinomas (2,20). The lung adenocarcinoma includes several histologic subtypes and is a highly heterogeneous tumor in terms of pathology, biology, and clinical behavior (21). As outcomes may vary even among patients with the same tumor type and stage, it is important to identify additional factors that may be used to detect patients with operable lung cancer who are at high risk for worse outcomes.
A p53 mutation inactivates the tumor suppressor gene, enabling the invasion, metastasis, proliferation, and cell survival of malignant cells (22). The genetic defect is frequently identified in more than 50% of resected NSCLCs (23-25). Conflicting reports have suggested that the p53 mutation is associated with decreased survival, no significant change in survival, or improved survival in lung cancer patients (26-29). IHC expression of p53 is generally regarded as indicative of a missense mutation of p53 gene (8).
The Ki-67 protein is a cellular marker for proliferation and is present during all phases of the cell cycle (G1, S, G2 and mitosis), but is absent in resting cells (G0) (30). A monoclonal antibody against the Ki-67 antigen has been utilized to assess tumor proliferation, and high expression level of Ki-67 in the tumor tissue is reported to be associated with poor prognosis in NSCLC (13,31). However, there was a report suggesting that Ki-67 level did not influence survival in NSCLC (32).
This retrospective study demonstrated that high expression level of Ki-67 in tumor cells was a poor prognostic factor for survival in patients with lung adenocarcinoma (P=0.018) (Figure 4B), especially in advanced stages (≥ stage III) (P=0.015) (Figure 6C). A similar association was also observed in 3-year overall survival among patients with stages I, II and ≥III (P=0.026) (Figure 7B) (12,13,31). The correlation of Ki-67 expression with the prognosis of lung cancer has been reported (13,14). Our study demonstrated that the high level of Ki-67 expression was associated with the lower overall survival by the Kaplan-Meier method (P=0.018). Moreover, smoking and poor tumor differentiation were correlated with the high level of Ki-67 expression in the tumors, upon multivariate analysis (P=0.051 and P=0.008) (Table 5).
However, no association was observed between p53 and the prognosis, in the group that combined all stages, or upon considering different stages, or 3-year overall survival outcomes (P>0.05) (Figures 4A,5A-C,7A). These findings differed from other studies in that p53 was associated with decreased survival (12,26-28). Conversely, the IHC expression of p53 in tumor cells tended to have a better outcome among patients with lung adenocarcinoma (28,29), although it was not statistically significant (P>0.05) (Figures 4A,5A,B). Discrepancies in the findings between previous reports and our results may result from the differences in patients’ characteristics between these studies.
In our study, the frequency of the p53 positivity by IHC was relatively high (71.0%), compared to other studies’ findings showing that p53 in the tumor occurred in the half of lung cancer cases (23-25). We collected the pure adenocarcinoma cases. Other studies, however, recruited NSCLC cases, including adenocarcinoma, squamous cell carcinoma and other non-small cell carcinoma. The study participants included a large fraction of women (47.1%), non-smokers (50.7%), and pathologic stage Ia cases (45.6%) (Table 1). Furthermore, the number of deaths included 11 patients (13.1%) with stage I (n=84), 5 (16.7%) with stage II (n=30) and 4 (18.2%) with stage ≥III (n=22) (Table 3). Overall, the 3-year cumulative survival rates were 90.6% for stage I, 80.7% for stage II, and 46.2% for stage ≥III, respectively. Thus, the lower number of deaths may statistically affect the survival analysis in patients with operable lung adenocarcinoma.
The 5-year survival rate in patients with pathologic stage I of NSCLC ranges from 57-67%, depending on stage Ia or Ib, and the location of tumor (33,34). However, Henschke et al. reported a 10-year survival rate of 94% in resected pathologic stage I (35). Sobue et al. showed that the 5-year survival rate of patients who underwent surgical resection for stage I lung cancers was 100% (36). In our study, the 3-year cumulative survival rates in pathologic stage I and II were relatively high (90.6% and 80.7%, respectively); these results may have been obtained owing to the surgeon’s high operative performance, leading to curative resection for lung cancer, as approximately half of the resected tumors (45.6%) were identified as localized disease, pathologic stage Ia.
Pretreatment lymphopenia has been revealed as a poor prognostic factor in patients with lung cancer (37). Of the 134 patients with follow-up, only 9 patients (6.7%) were diagnosed with lymphopenia at the diagnosis of lung cancer. Owing to the small fraction of lymphopenia cases, it is difficult to attribute any clinical significance, however, patients without lymphopenia showed statistically lower survival than those with lymphopenia (P=0.028).
Zhang et al. identified 20 out of 21 known lung cancer gene mutations were found in all regions of the same tumor from 11 localized lung adenocarcinomas; post-surgical relapse was significantly associated with most of the subclonal mutations that cause tumor heterogeneity in their primary tumors (38). In this study, there were 8 cases (9.5%) of recurrence or progression in stage I, 5 (16.7%) in stage II, and 7 (35%) in stage IIIa (Table 3). Although a p53 or high level Ki-67 expression may not predict early recurrence after surgery in our findings, it bears consideration that close follow-up should be performed in patients who underwent potentially curative resection of lung adenocarcinoma.
The limitations of our study include a small sample size, and the retrospective analysis of data. This may yield some bias in survival rates owing to uncensored data. Furthermore, the detection of p53 and Ki-67 expression was based on the IHC assay, which limits the diagnostic accuracy. The p53 may be overestimated in the tumor cells by the IHC assay instead of the molecular technique, as the p53 antibody clone DO7 used in this study recognizes both the wild-type and mutant p53 proteins. Consequently, weak or patchy staining with p53 antibodies may be considered positive results.
In conclusion, high expression level of Ki-67 in the tumor was found to be a poor prognostic marker in patients with higher-stage lung adenocarcinoma, which might potentially identify a subgroup of subjects with a higher risk of worse outcomes after surgery. We propose that the evaluation of Ki-67 expression using an IHC assay is important and useful in routine practice. However, as IHC analysis for p53 showed no clinical significance, further investigation is needed to verify its prognostic role in lung adenocarcinoma.
Acknowledgements
The authors would like to thank Ye Ran Choi, P.A., Hwan Hee Son, P.A. and Joon Young Park, P.A. for their assistance with data collection.
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat 2011;43:1-11. [PubMed]
- Travis WD, Garg K, Franklin WA, et al. Evolving concepts in the pathology and computed tomography imaging of lung adenocarcinoma and bronchioloalveolar carcinoma. J Clin Oncol 2005;23:3279-87. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12. [PubMed]
- Nigro JM, Baker SJ, Preisinger AC, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature 1989;342:705-8. [PubMed]
- Bullwinkel J, Baron-Lühr B, Lüdemann A, et al. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol 2006;206:624-35. [PubMed]
- Iggo R, Gatter K, Bartek J, et al. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet 1990;335:675-9. [PubMed]
- Shiba M, Kohno H, Kakizawa K, et al. Ki-67 immunostaining and other prognostic factors including tobacco smoking in patients with resected nonsmall cell lung carcinoma. Cancer 2000;89:1457-65. [PubMed]
- Dubinski W, Leighl NB, Tsao MS, et al. Ancillary testing in lung cancer diagnosis. Pulm Med 2012;2012:249082.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Maddau C, Confortini M, Bisanzi S, et al. Prognostic significance of p53 and Ki-67 antigen expression in surgically treated non-small cell lung cancer: immunocytochemical detection with imprint cytology. Am J Clin Pathol 2006;125:425-31. [PubMed]
- Haga Y, Hiroshima K, Iyoda A, et al. Ki-67 expression and prognosis for smokers with resected stage I non-small cell lung cancer. Ann Thorac Surg 2003;75:1727-32; discussion 1732-3.
- Hommura F, Dosaka-Akita H, Mishina T, et al. Prognostic significance of p27KIP1 protein and ki-67 growth fraction in non-small cell lung cancers. Clin Cancer Res 2000;6:4073-81. [PubMed]
- Ermiah E, Buhmeida A, Abdalla F, et al. Prognostic value of proliferation markers: immunohistochemical ki-67 expression and cytometric s-phase fraction of women with breast cancer in libya. J Cancer 2012;3:421-31. [PubMed]
- Molino A, Micciolo R, Turazza M, et al. Ki-67 immunostaining in 322 primary breast cancers: associations with clinical and pathological variables and prognosis. Int J Cancer 1997;74:433-7. [PubMed]
- Pinto AE, André S, Pereira T, et al. Prognostic comparative study of S-phase fraction and Ki-67 index in breast carcinoma. J Clin Pathol 2001;54:543-9. [PubMed]
- McCarty KS Jr, Miller LS, Cox EB, et al. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 1985;109:716-21. [PubMed]
- Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol 2007;25:561-70. [PubMed]
- Kim JH, Park K, Yim SH, et al. Genome-wide association study of lung cancer in Korean non-smoking women. J Korean Med Sci 2013;28:840-7. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung adenocarcinoma in resected specimens: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:685-705. [PubMed]
- Ferreira CG, Tolis C, Giaccone G. p53 and chemosensitivity. Ann Oncol 1999;10:1011-21. [PubMed]
- Ahrendt SA, Chow JT, Yang SC, et al. Alcohol consumption and cigarette smoking increase the frequency of p53 mutations in non-small cell lung cancer. Cancer Res 2000;60:3155-9. [PubMed]
- Athanassiadou P, Dosios T, Petrakakou E, et al. p53 and bcl-2 protein expression in non-small-cell lung carcinoma. Diagn Cytopathol 1998;19:255-9. [PubMed]
- Greenblatt MS, Bennett WP, Hollstein M, et al. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res 1994;54:4855-78. [PubMed]
- Skaug V, Ryberg D, Kure EH, et al. p53 mutations in defined structural and functional domains are related to poor clinical outcome in non-small cell lung cancer patients. Clin Cancer Res 2000;6:1031-7. [PubMed]
- Tomizawa Y, Kohno T, Fujita T, et al. Correlation between the status of the p53 gene and survival in patients with stage I non-small cell lung carcinoma. Oncogene 1999;18:1007-14. [PubMed]
- Top B, Mooi WJ, Klaver SG, et al. Comparative analysis of p53 gene mutations and protein accumulation in human non-small-cell lung cancer. Int J Cancer 1995;64:83-91. [PubMed]
- Lee JS, Yoon A, Kalapurakal SK, et al. Expression of p53 oncoprotein in non-small-cell lung cancer: a favorable prognostic factor. J Clin Oncol 1995;13:1893-903. [PubMed]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000;182:311-22. [PubMed]
- Tabata K, Tanaka T, Hayashi T, et al. Ki-67 is a strong prognostic marker of non-small cell lung cancer when tissue heterogeneity is considered. BMC Clin Pathol 2014;14:23. [PubMed]
- Cagini L, Monacelli M, Giustozzi G, et al. Biological prognostic factors for early stage completely resected non-small cell lung cancer. J Surg Oncol 2000;74:53-60. [PubMed]
- Ou SH, Zell JA, Ziogas A, et al. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 2007;110:1532-41. [PubMed]
- Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007;132:193-9. [PubMed]
- International Early Lung Cancer Action Program Investigators, Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [PubMed]
- Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol 2002;20:911-20. [PubMed]
- Lissoni P, Brivio F, Fumagalli L, et al. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers 2004;19:135-40. [PubMed]
- Zhang J, Fujimoto J, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 2014;346:256-9. [PubMed]


