|
Review Article
DNA topoisomerase I drugs and radiotherapy for lung cancer
Allan Y. Chen1,2, Patricia M. T. Chen2, Yi-Jen Chen3
1Department of Radiation Oncology, The Permanente Medical Group, Roseville, USA; 2School of Medicine, University of California, Davis,
USA; 3Department of Radiation Oncology, City of Hope Medical Center, Duarte, USA
Corresponding to: Allan Y. Chen. Department of Radiation Oncology, The
Permanente Medical Group, Roseville, CA 95678, USA. Email: allan.y.chen@kp.org.
|
|
Abstract
Lung cancer represents the most common cause of cancer-related mortality in the United States and around the world. DNA
topoisomerase I (TOP1) drugs such as irinotecan and topotecan represent a unique class of chemotherapeutic agents that
exhibit not only potent cytotoxic effect, but also tumor-selective radiation-sensitizing effect. The mechanism of cytotoxicity
and radiation sensitization by TOP1 drugs has been intensely investigated. Modern radiotherapy, aided by improved
imaging and treatment delivery technology, is capable of targeting tumors more precisely, while sparing surrounding critical
structures. Clinical trials with camptothecin derivatives and radiotherapy have been conducted in lung cancers. Combined
modality therapy with TOP1 drugs and radiotherapy offers a new frontier for lung cancer therapy. We review the present
state of TOP1-targeted chemotherapy and modern radiotherapy for lung cancer.
Key words
DNA topoisomerase I (TOP1); camptothecin derivative; radiation therapy; lung cancer; radiation sensitizer
J Thorac Dis 2012;4(4):390-397. DOI: 10.3978/j.issn.2072-1439.2012.07.12 |
|
Introduction
In 2012, more than 1.6 million new cancer cases and close
to 0.6 million deaths (about 35% of new cases) from cancer
are projected to occur in the United States ( 1). Albeit small
improvement, it has been noted that the overall cancer death
rates from 2004 to 2008 have decreased by 1.8% per year in men
and by 1.6% per year in women ( 1). Lung cancer represents the
most common cause of cancer-related mortality in the United
States and around the world. Despite medical advances, lung
cancer still accounts for more than 150,000 deaths annually in
the United States ( 1). While surgical intervention with lobectomy and mediastinal
lymph node dissection is considered the standard of treatment
for early-stage non-small cell lung cancer (NSCLC) ( 2), systemic
chemotherapy and local field radiotherapy are the mainstay
therapies for small cell lung cancer (SCLC) and advancedstage
NSCLC ( 3, 4). Radiotherapy plays important roles in both
curative and palliative treatment for lung cancer patients ( 5). An estimated 76% of lung cancer patients might benefit from
radiotherapy ( 6). Guided by much improved imaging modalities,
new radiotherapy technologies make possible the delivery of
highly conformal radiation to the tumor target with precision
( 7, 8). However, given the similar radiation sensitivities shared by
most solid tumors and their counterpart normal tissues and the
fact that radiation beams inevitably irradiating through tissues
surrounding the target, the efficacy of radiotherapy remains
largely constrained by the potential radiation-induced toxicities
upon the normal tissues ( 9, 10). Combining chemotherapy with radiotherapy represents a
key oncology strategy for a more comprehensive attack toward
cancers. Combination chemoradiotherapy has been shown to
improve treatment outcome for various solid tumor malignancies
including lung cancer. By treating overt or microscopic
metastatic lesions, systemic chemotherapy complements local
primary tumor control provided by radiotherapy. In addition, a
number of chemotherapeutic drugs exhibit radiation-sensitizing
activity and are capable of enhancing efficacy of radiotherapy
targeting at primary tumor ( 10, 11). DNA topoisomerase I
(TOP1)-targeted camptothecin derivatives represent a novel class
of anticancer agents that exhibit potent cytotoxicity ( 12-14), as well
as tumor-selective radiation-sensitizing effect toward a variety
of solid tumors ( 14-16). Combination therapy with TOP1
drugs and radiotherapy has great potential to improve treatment
efficacy and decrease normal tissue toxicities. Enhancement of
radiotherapy with TOP1 drugs offers a new frontier for cancer
therapy. In this article, we review the present state of TOP1-targeted chemotherapy and modern radiotherapy from basic
science to clinical applications in lung cancers. |
|
DNA topoisomerase I as a therapeutic target
DNA topoisomerases I (TOP1) and II (TOP2) are essential
nuclear enzymes that catalyze the interchange of DNA double-helix
between various topological states. Human TOP1 is involved in RNA
transcription, DNA replication and maintaining genome stability
by regulating the supercoiling state of DNA (reviewed in 17). The
cellular level of TOP1 is up-regulated in both slow and rapidly
proliferating tumor cells ( 18, 19). This provides a scientific basis
for tumor-selective targeting by TOP1 drugs. A number of anticancer compounds, including camptothecins ( 13),
DNA minor groove-binders ( 20) and indolocarbazole derivatives
( 21, 22), have been demonstrated to exert their cytotoxic effect
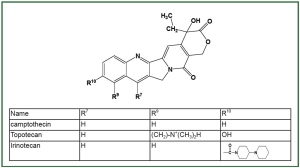
through TOP1. Camptothecin and its derivatives ( Figure 1) are
the currently best-characterized TOP1-targeting anticancer drugs.
Topotecan (Hycamtin) and irinotecan (Camptosar, CPT-11) were
initially approved by the FDA for treatment of recurrent ovarian
and colon cancers, respectively ( 23). Based on their demonstrated
efficacies in clinical trials, the clinical usage of topotecan and
irinotecan has been rapidly expanded to include other cancers
such as NSCLC and SCLC ( 24). |
|
DNA topoisomerase I drugs as radiation sensitizers
DNA is the critical molecular target for ionizing radiation ( 25), with
double-strand DNA break being the major type of lethal lesion ( 26).
Generally, radiation sensitizers may enhance radiation cytotoxicity
by means of increasing amount of DNA damage, inhibiting repair
of DNA damage or re-distributing cells into radiation-sensitive
phases of the cell cycle, such as the G2/M and G1 phases ( 27).
In addition to diverse mechanisms of action, factors affecting
bioavailability at the target site are major determinants for the
effectiveness of different radiation sensitizers. Recent advances in studying the radiation-sensitizing effect
of TOP1 drugs in preclinical systems have contributed greatly
to the clinical application of combined modality therapy
with radiation and TOP1-targeted drugs ( 14). For example,
camptothecin derivatives were shown to induce radiation
sensitization in cultured human breast cancer MCF-7 cells in
a schedule-dependent manner that requires drug treatment
prior to, but not following radiation ( 15). This observation
indicates the importance of treating patients with TOP1 drugs
prior to delivery of radiotherapy. Based on studies using DNA
polymerase inhibitors and phase-specific cells sorted by cellcycle
sorting techniques, the induction of TOP1-mediated
radiation sensitization in mammalian cells was shown to be an
S-phase-specific event that requires active DNA synthesis ( 28).
This finding indicates a probable therapeutic advantage of TOP1
drugs in selectively radiosensitizing proliferating cancer cells that
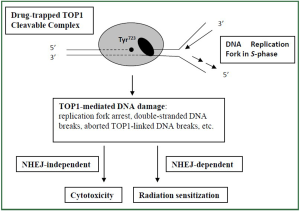
are actively synthesizing DNA. Eukaryotic cells have evolved two major repair pathways for
DNA double-strand breaks (DSB) including the homologous
recombination and the non-homologous end-joining (NHEJ)
pathways ( 29). Inactivation of the NHEJ pathway was
demonstrated to significantly enhance TOP1-mediated radiation
sensitization, but not cytotoxicity, in preclinical cultured
mammalian cells ( 28). This study suggests TOP1 drugs may
induce a unique NHEJ-dependent radiation sensitization pathway
that is distinctive from their cytotoxicity pathway ( Figure 2). It is conceivable that inhibitors of NHEJ pathway can be used clinically
to enhance radiosensitizing effect of TOP1 drugs. |
|
Modern precision radiotherapy in early stage non-small cell lung cancer
With the innovations of stereotactic radiosurgery, three
dimensional (3-D) radiation treatment planning, IMRT
(intensive modulated radiation therapy), VMAT (volumetric
modulated arc therapy) and image-guided radiation therapy
(IGRT), radiotherapy has experienced an unprecedented
technical advancement in the recent 20 years ( 7, 8). Fourdimensional
CT (4D-CT) represents a major breakthrough that
allows accurate determination of internal target volume (ITV)
for mobile lung tumors in individual patients ( 30, 31). Conceptually derived from cranial stereotactic radiosurgery,
stereotactic body radiation therapy (SBRT) has been emerging
as an excellent alternative for medically inoperable early stage
NSCLC patients. In lung SBRT, a total of 45-50 Gy of hypofractionated
radiotherapy is delivered in 3-5 fractions over a 10-
20 days’ duration. Available data have shown an impressive 80-
95% local tumor control at 2-5 years and good lung function
preservation ( 8, 32). The recently published RTOG 0236 phase
II study demonstrated 3-years 98% local tumor control and
56% survival ( 7). These results are comparable to the 5-year
53% survival with surgical resection, based on a compiled result
from thousands of patients in the International Association
for the Study of Lung Cancer Staging Project ( 8). Though
with demonstrated impressive effectiveness, the usage of any
newly developed medical technology such as SBRT requires
special caution. A recent report of fatal central-airway necrosis
in a patient with a centrally-located lesion treated with SBRT
highlights the importance of long-term follow-up for SBRTtreated
patients ( 33). Modeling exercises demonstrate that significant increases
in biologically equivalent dose may be achieved with the
addition of radiation sensitizers to hypo-fractionated
radiotherapy ( 34). How to incorporate the cytotoxic and
radiosensitizing effects of TOP1 drugs with modern precision
hypo-fractionated radiotherapy for lung cancer remains to be
explored. |
|
Clinical trials of camptothecin derivatives and radiotherapy in lung cancer
A large number of clinical trials have demonstrated efficacy of
TOP1-targeted camptothecin derivatives in the treatment of
NSCLC, as well as SCLC.
Clinical chemotherapy trials of camptothecin derivatives in
SCLC and NSCLC
Topotecan is currently a standard second-line therapy for
patients with SCLC ( 35, 36). Single agent regimen with daily
intravenous infusion of 1.5 mg/m 2 in 30 min for first 5 days of
a 21-days cycle demonstrated comparable outcomes as a threedrug
combination with cyclophosphamide, doxorubicin and
vincristine for recurrent SCLC patients ( 37). Topotecan is FDA
approved for patients with SCLC who relapse after first line
chemotherapy ( 4). Combination irinotecan and cisplatin has been shown to
improve survival than the standard regimen of etoposide and
cisplatin for extensive-stage SCLC in a Japan Clinical Oncology
Group (JCOG) phase III study ( 38). Interestingly, a subsequent
larger North American SWOG S0124 trial only demonstrated
statistically comparable efficacies for both regimens ( 39).
Irinotecan-containing regimens were noted to cause less severe
hematological side effects in neutropenia and thrombocytopenia,
but more severe gastroenterological toxicities in vomiting and
diarrhea than the etoposide and cisplatin regimen. Noteworthy
mentioning, a laboratory correlated pharmacogenomics analysis
of the SWOG S0124 trial indicated that ABCB1 (C3435T) T/
T (membrane transport) and UGT1A1 (G-3156A) A/A (drug
metabolism) genotypes are related to the irinotecan-related
diarrhea and neutropenia, respectively. In a recent meta-analysis
of six trials involving about 1,500 chemo-naïve extensive-stage
SCLC patients, irinotecan and platinum combination regimens
did demonstrate greater overall survival than etoposide and
cisplatin combination ( 40). Studies showed that irinotecan is an active chemotherapeutic
agent for metastatic NSCLC with acceptable toxicities. As a
single agent or in combination with cisplatin, irinotecan has
demonstrated promising efficacy with up to 30% response
rate and a median survival of 50 weeks in previously untreated
NSCLC patients ( 41, 42). Clinical chemoradiation trials of camptothecin derivatives in
NSCLC
Many clinical studies have demonstrated the feasibility
and efficacy of TOP1 drugs in combination with thoracic
radiotherapy for locally advanced NSCLC ( Table 1).
| Table 1. Clinical trials of concurrent TOP1 drugs with thoracic radiotherapy for NSCLC. |
| Study description |
No. of patients |
Chemotherapy regimen |
Radiation dose |
Toxicity |
Response rate |
|---|
| Phase II (43) |
24 |
Irinotecan; 60-70 mg/m2, weekly ×6 |
60 Gy |
Neutropenia esophagitis pneumonitis |
79% (19 PR) |
| Phase I/II (44) |
26 |
Irinotecan; 30-60 mg/m2, weekly ×6 |
60 Gy |
Esophagitis pneumonitis diarrhea |
77% (3 CR, 17 PR) |
| Phase II (45) |
12 |
Irinotecan; 30-50 mg/m2, weekly ×6 |
60 Gy |
Nausea/vomiting esophagitis |
58% (7 PR) |
| Phase I/II (46) |
26 |
Irinotecan; 45 mg/m2, weekly ×6 |
60 Gy |
Diarrhea esophagitis pneumonitis |
75% (2 CR, 16 PR) |
| Phase II (47) |
68 |
Irinotecan + Cisplatin, Induction ×2, then weekly |
60 Gy |
Neutropenia esophagitis pneumonitis |
63% (4 CR, 39 PR) |
| Phase I/II (48) |
12 |
Irinotecan + Cisplatin, every 4 weeks ×3 |
60 Gy |
Neutropenia diarrhea |
67% (8 PR) |
| Phase I/II (49) |
30 |
Irinotecan weekly, 30-60 mg/m2, Carboplatin daily for 4 weeks |
60 Gy |
Neutropenia esophagitis pneumonitis |
60% (3 CR, 15 PR) |
| Phase I/II (50) |
12 |
Topotecan; 0.5-1 mg/m2, Days 1-5, 22-26 |
60 Gy |
Nausea neutropenia esophagitis |
17% (2 CR) |
| Phase I/II (51) |
24 |
Topotecan; 0.4 mg/m2, Daily, 21-42 days |
30-60 Gy |
Neutropenia esophagitis |
43% (9 PR, 6 SD) |
| NSCLC = non-small cell lung cancer; CR = complete response; PR = partial response; SD = stable disease. |
Weekly injection of irinotecan with concurrent thoracic
radiotherapy for locally advanced NSCLC has been studied
in a number of phase I and II trials. In these studies, MTD of
intravenous injection of irinotecan, administered weekly for
6 weeks concurrently with thoracic radiotherapy to 60 Gy,
was shown to be from 40 to 60 mg/m 2 ( 43-46). Dose-limiting
toxicities included esophagitis, pneumonitis, diarrhea, nausea
and vomiting. A generally good response rate of 58% to 79% was
reported ( 43-46). Irinotecan, in combination w ith c isplat in-based
chemotherapy and daily thoracic radiotherapy, has also been
tested in stage III NSCLC patients. A phase I/II trial with
irinotecan and cisplatin chemotherapy (4-weeks interval, a total
of 3 cycles), and 60 Gy of thoracic radiotherapy was conducted
by Yokoyama et al. ( 48). Leukopenia and diarrhea were the
dose-limiting toxicities, and an overall response rate of 67%
was reported. In another phase I/II trial with 30 patients with
unresectable stage III NSCLC, weekly irinotecan and daily
carboplatin (20 mg/m 2/day, 5 days weekly for 4 weeks) were
administered with concurrent 60 Gy of thoracic radiotherapy ( 49).
The MTD of irinotecan was determined to be 60 mg/m 2, with
pneumonitis, nausea and vomiting as dose-limiting toxicities. An
objective response rate of 60% was observed in the study. Single agent topotecan, given by daily bolus injection on days
1 to 5, and days 22 to 26, was dose-escalated with concurrent
daily thoracic radiotherapy in a Phase I study for 12 patients
with unresectable locally advanced NSCLC ( 50). Dose-limiting
toxicities included esophagitis and neutropenia, and the MTD
was 0.5 mg/m 2/day. A response rate of 17% with 2 complete
responses was reported. Another phase I study was conducted
with escalating both thoracic radiotherapy and infusion duration
of topotecan at constant dose 0.4 mg/m 2/day. The radiation dose
(30, 40 and 60 Gy) and topotecan infusion duration (21, 28, 35
and 42 days) were escalated in an alternating fashion at different
dose levels ( 51). Studies reported well-tolerated side effects
and recommended 60 Gy thoracic radiotherapy and 42-day
duration of topotecan 0.4 mg/m 2/day as the phase II regimen.
A good 43% response rate was reported for a total of 24 patients,
including 22 patients with NSCLC. Clinical chemoradiation trials of camptothecin derivatives in
SCLC
A number of clinical phase I/II studies with camptothecins,
in combination with cisplatin-based chemotherapy and
concomitant thoracic radiotherapy, have shown promising
efficacy for SCLC ( Table 2). Oka et al. conducted a phase I
study of irinotecan and cisplatin with concurrent split-course
radiotherapy in limited-stage SCLC ( 52). Patients were treated
with four cycles of irinotecan (days 1, 8 and 15) and cisplatin (day 1)
at 28-days intervals, together with a total of 60 Gy thoracic
radiotherapy commenced on day 2 of each chemotherapy cycle,
with 20 Gy in 10 daily fractions administered in the first, second
and third cycles of chemotherapy. The MTDs of irinotecan
and Cisplatin were determined to be at 50 and 60 mg/m 2,
with general fatigue listed as dose-limiting toxicity. An overall
response rate of 94% with 4 complete responses was reported for
16 evaluable patients ( 52).
| Table 2. Clinical trials of concurrent TOP1 drugs with thoracic radiotherapy for SCLC. |
Study
description |
No. of patients |
Chemotherapy
regimen |
Radiation dose |
Toxicity |
Response |
|---|
| Phase I (52) |
17 (all LS) |
Irinotecan + cisplatin |
60 Gy in 3 split courses of 20 Gy in 10 daily fractions |
Fatigue |
94% (4 CR, 11 PR) |
| Phase II (53) |
100 (43 LS, 57 ES) |
Topotecan + carboplatin +paclitaxel |
45 Gy in 25 daily fractions for LD |
Neutropenia, thrombocytopenia, fatigue |
LS -93% ES -88% |
| Phase II (54) |
78 (all LS) |
Topotecan + carboplatin +paclitaxel |
61.2 Gy in 34 daily fractions |
Neutropenia, thrombocytopenia, fatigue fatal pneumonitis |
51% CR SV: 20 months |
| SCLC = small cell lung cancer; LS = limited-stage; ES = extensive-stage; CR = complete response; SV = median survival. |
A paclitaxel/carboplatin/topotecan regimen was evaluated as the first-line treatment in a phase II study consisting of 43
limited-stage and 57 extensive-stage SCLC patients ( 53). During
the 4 courses of chemotherapy at 21-day intervals (paclitaxel
135 mg/m 2, 1-hour IV infusion, day 1; carboplatin AUC 5.0
IV, day 1; topotecan 0.75 mg/m 2 IV, days 1-3), patients with
limited-stage SCLC also received 45 Gy of thoracic radiotherapy
in 25 daily fractions, beginning week 6 of chemotherapy. Overall
response rate of 90% (extensive-stage 88%; limited-stage 93%)
with toxicities including neutropenia, thrombocytopenia
and fatigue was reported. In a subsequent study conducted
by the same group of researchers, the paclitaxel/carboplatin/
topotecan regimen was combined with a higher 61.2 Gy of thoracic
radiotherapy to treat 78 patients with limited-stage SCLC ( 54).
A high 51% complete response rate and median survival of
20 months were reported for 68 evaluable patients after a
short median follow-up of 12 months. However, in addition to
high incidence of neutropenia, thrombocytopenia and fatigue,
three treatment-related deaths (2 radiation pneumonitis; 1
pneumonia/neutropenia) were reported ( 54). Irinotecan and topotecan chemotherapy in brain metastases
from SCLC
Brain metastases occur commonly in SCLC patients. The risk
of brain metastases in SCLC patients ranges from 18 to 25%
at presentation, to 50% during the 2-year course of disease
( 55, 56). Brain metastases from SCLC are traditionally treated
by radiotherapy, since most chemotherapeutic agents exert low
efficacies due to factors such as the blood-brain barrier (BBB)
that prevents penetration of anticancer drugs into the central
nervous system. Nevertheless, recent data suggest that the BBB
may be disrupted with the existence of brain metastasis and,
consequentially, permeable to anticancer drugs ( 57). Irinotecan and topotecan, appear to penetrate the BBB better
and exert anticancer activity for brain metastases ( 58-60). A
multicenter phase II study was conducted to evaluate the
efficacy of single agent topotecan in 30 patients with SCLC
who relapsed with symptomatic brain metastases after previous
chemotherapy (30 patients) and whole brain radiotherapy
(8 patients). Twenty patients received the initially planned
topotecan 1.5 mg/m 2 as a 30-min intravenous infusion for 5
consecutive days every 3 weeks, with the last 8 patients received
reduced dose topotecan 1.25 mg/m 2 due to the observed
thrombocytopenia ( 58). An impressive 33% overall response
rate, including 3 complete responses and 7 partial responses,
and well-tolerated hematological side effects was reported ( 58).
In another phase II trial, 80 patients with metastatic or relapsed
SCLC were treated with irinotecan 200 mg/m 2 (chemotherapy
naïve patients) or 150 m 2 (previously chemotherapy treated
patients), in combination of carboplatin AUC of 5, every 21 days for
6 cycles. An analysis of 14 assessable patients with brain metastases
in this study revealed an impressive overall response rate of
65% after 2 cycles of chemotherapy, and a median survival of 6
months ( 60). |
|
Conclusions
Important information such as the sequence of chemoradiation
combination and important determinants for TOP1-
mediated radiation sensitization has been obtained through
characterization of camptothecin derivatives. Tumor-selective
targeting due to the up-regulated level of TOP1 in cancer cells
and S-phase specific mechanism may contribute to therapeutic
advantages of anticancer chemoradiotherapy with TOP1 drugs.
As the understanding of molecular pharmacology progressively
influences treatment strategy, a better elucidation of the
mechanism of TOP1 drug will lead to development of better
chemoradiation regimens.
Clinical trials have demonstrated that irinotecan and
topotecan are active anticancer drugs with potent cytotoxicity
and radiation sensitization activity for lung cancer. The optimal
combination with other chemotherapy drugs, as well as
scheduling between TOP1 drugs and thoracic radiotherapy
for the treatment of lung cancer remains to be defined. The
promising role of irinotecan and topotecan in treating SCLC
patients with brain metastases requires further investigation.
|
|
Acknowledgements
The authors would like to thank Ms. Michelle B. Chen for editing
assistance in the preparation of this manuscript.
Disclosure: The authors declare no conflict of interest.
|
|
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin
2012;62:10-29.
- Wright G, Manser RL, Byrnes G, et al. Surgery for non-small cell lung
cancer: systematic review and meta-analysis of randomised controlled
trials. Thorax 2006;61:597-603.
- Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet
2011;378:1727-40.
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer.
Lancet 2011;378:1741-55.
- Principles and Practice of Radiation Oncology. 5th ed., Perez and Brady
(eds.), Philadelphia, J. B. Lippincott Co., 2004.
- Delaney G, Barton M, Jacob S, et al. A model for decision making for the
use of radiotherapy in lung cancer. Lancet Oncol 2003;4:120-28.
- “Image-Guided Radiotherapy of Lung Cancer”, Cox JD, Chang JY &
Komaki R, (ed.), informa, Health care Communications, 2007. Available
online: http://informahealthcare.com/doi/pdf/10.3109/9780849387821
- Kavanagh BD, Timmerman RD. eds. “Stereotactic Body Radiation Therapy”. Philadelphia, Lippincott Williams Wilkins. 2005.
- Hall EJ, Giaccia AJ. eds. Radiobiology for the Radiologist, 6th ed.
Philadelphia, J. B. Lippincott Co., 2006.
- John, Flam, Legha and Phillips. eds. “Chemoradiation: an integrated approach
to cancer treatment”, 1st ed., Philadelphia, J. B. Lippincott Co., 1995.
- McGinn CJ, Shewach DS, Lawrence TS. Radiosensitizing nucleotides. J
Natl Cancer Inst1996;88:1193-1203.
- Chen AY, Liu LF. DNA topoisomerases: essential enzymes and lethal
targets. Annu Rev Pharmacol Toxicol 1994;34:191-218.
- Liehr, Giovanella, Verschraegen. eds. The Camptothecins - unfolding their
anticancer potential., New York, Ann. N. Y. Acad. Sci., 2000.
- Chen AY, Chou R, Shih S-J, et al. Enhancement of Radiotherapy with DNA
Topoisomerase I-targeted Drugs. Crit Rev Oncol Hematol 2004;50:111-119.
- Chen AY, Okunieff P, Pommier Y, et al. Mammalian DNA Topoisomerase
I Mediates the Enhancement of Radiation Cytotoxicity by Camptothecin
Derivatives. Cancer Res 1997;57:1529-36.
- Chen AY, Choy H, Rothenberg ML. DNA Topoisomerase I-targeting drugs
as radiation sensitizers. Oncology 1999;13:39-46.
- Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective.
Nat Rev Mol Cell Biol 2002;3:430-40.
- Giovanella BC, Stehlin JS, Wall ME, et al. DNA topoisomerase I--
targeted chemotherapy of human colon cancer in xenografts. Science
1989;246:1046-8.
- Pantazis P, Early JA , Kozielski A J, et al. Regression of human
breast carcinoma tumors in immunodeficient mice treated with
9-nitrocamptothecin: differential response of nontumorigenic and
tumorigenic human breast cells in vitro. Cancer Res 1993;53:1577-82.
- Chen AY, Yu C, Gatto B, et al. DNA minor groove-binding ligands: a
different class of mammalian DNA topoisomerase I inhibitors. Proc Natl
Acad Sci USA 1993;90:8131-35.
- Yamashita Y, Fujii N, Murakata C, et al. Induction of mammalian DNA
topoisomerase I mediated DNA cleavage by antitumor indolocarbazole
derivatives. Biochemistry 1992;31:12069-75.
- Bailly C, Riou JF, Colson P, et al. DNA cleavage by topoisomerase I in the
presence of indolocarbazole derivatives of rebeccamycin. Biochemistry
1997;36:3917-29.
- Takimoto CH, Wright J, Arbuck SG. Clinical applications of the
camptothecins. Biochem Biophys Acta 1998;1400:107-19.
- Choy H, Macrae R. Irinotecan and Radiation in Combined-Modality
Therapy for Solid Tumors. Oncology 2001;15:22-28.
- Munro TR. The relative radiosensitivity of the nucleus and cytoplasm of
the Chinese hamster fibroblasts. Radiat Res 1970;42:451-70.
- Radford IR. Evidence for a general relationship between the induced
level of DNA double-strand breakage and cell-killing after X-irradiation
of mammalian cells. Int J Radiat Biol Relat Stud Phys Chem Med
1986;49:611-20.
- Herscher LL, Cook JA, Pacelli R, et al. Principles of chemoradiation:
theoretical and practical considerations. Oncology 1999;13:11-22.
- Shih S-J, Erbele T, Chen AY. Ku86 Modulates DNA Topoisomerase
I-mediated Radiosensitization, but not cytotoxicity, in Mammalian Cells.
Cancer Res 2005;65: 9194-99.
- Khanna KK, Jackson SP. DNA double strand breaks: signaling, repair and
the cancer connection. Nat Genet 2001;27:247-54.
- Vedam SS, Keall PJ, Kini VR, et al. Acquiring a four-dimensional computed
tomography dataset using an external respiratory signal. Phys Med Biol
2003;48:45-62.
- Chen AY, Chen MB, Chen YJ. A millimeter miss is as good as a thousand
miles: The role of accurate target localization in lung stereotactic body
radiation therapy. J Thorac Dis 2012;4:109-11.
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic
radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated
results of 257 patients in a Japanese multi-institutional study. J Thorac
Oncol 2007;2:S94-100.
- Corradetti MN, Haas AR, Rengan R. Central-Airway Necrosis after
Stereotactic Body-Radiation Therapy. N Engl J Med 2012;366:2327-29.
- Ohri N, Dicker AP, Lawrence YR. Can drugs enhance hypofractionated
radiotherapy? A novel method of modeling radiosensitization using in vitro
data. Int J Radiat Oncol Biol Phys 2012;83:385-93.
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing
supportive care alone with supportive care with oral topotecan in patients
with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7.
- Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared
with intravenous topotecan as second-line therapy in small-cell lung cancer.
J Clin Oncol 2007;25:2086-92.
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus
cyclophosphamide, doxorubicin, and vincristine for the treatment of
recurrent small-cell lung cancer. J Clin Oncol 1999;17:658-67.
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin
compared with etoposide plus cisplatin for extensive small-cell lung cancer.
N Engl J Med 2002;346:85-91.
- Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin
compared with etoposide/cisplatin in extensive-stage small-cell lung
cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin
Oncol 2009;27:2530-5.
- Jiang J, Liang X, Zhou X, et al. A meta-analysis of randomized controlled
trials comparing irinotecan/platinum with etoposide/platinum in patients
with previously untreated extensive-stage small cell lung cancer. J Thorac
Oncol 2010;5:867-73.
- Fukuoka M, Niitani H, Suzuki A, et al. A phase II study of CPT-11, a new
derivative of camptothecin for previously untreated non-small cell lung
cancer. J Clin Oncol 1992;10:16-20.
- Negoro S, Masuda N, Takada Y, et al. Randomized phase III trial of
irinotecan combined with cisplatin for advanced non-small-cell lung
cancer. Br J Cancer 2003;88:335-41.
- Saka H, Shimokata K, Yoshida S, et al. Irinotecan (CPT-11) and concurrent
radiotherapy in locally advanced non-small cell lung cancer (NSCLC): A
phase II study of Japan Clinical Oncology Group (JCOG9504). Proc Am
Soc Clin Oncol 1997;16:447.
- Takeda K, Negoro S, Okishio K, et al. Phase I/II study of weekly irinotecan
and concurrent radiation therapy for locally advanced non-small cell lung
cancer. Br J Cancer 1999;79:1462-67.
- Choy H, Chakravarthy A, Devore RF, et al. Weekly irinotecan and concurrent radiation therapy for stage III unresectable NSCLC. Oncology
2000;14:43-46.
- Kodoh S, Kurihara N, Okishio K, et al. A phase I/II study of weekly
irinotecan (CPT- 11) and simultaneous thoracic radiotherapy (TRT) for
unresectable locally advanced non-small cell lung cancer (NSCLC). Proc
Am Soc Clin Oncol 1996;15:1102.
- Takeda K, Negoro S, Tanaka M, et al. A phase II study of cisplatin and
irinotecan as induction chemotherapy followed by concomitant thoracic
radiotherapy with weekly low-dose irinotecan in unresectable, stage III,
non-small cell lung cancer: JCOG 9706. Jpn J Clin Oncol 2011;41:25-31.
- Yokoyama A, Kurita Y, Saijo N, et al. Dose-finding study of irinotecan and
cisplatin plus concurrent radiotherapy for unresectable stage III non-smallcell
lung cancer. Br J Cancer 1998;78:257-62.
- Yamada M, Kudoh S, Fukuda H, et al. Dose escalation study of weekly
irinotecan and daily carboplatin with concurrent thoracic radiotherapy for
unresectable stage III non-small cell lung cancer. Br J Cancer 2002;87:258-63.
- Graham MV, Jahanzeb M, Dresler CM, et al. Results of a trial with
topotecan dose escalation and concurrent thoracic radiation therapy for
locally advanced, inoperable nonsmall cell lung cancer. Int J Radiat Oncol
Biol Phys 1996;36:1215-20.
- Chachoua A, Hochster H, Steinfeld A, et al. Feasibility of seven weeks
concomitant topotecan (T) continuous infusion with thoracic radiation.
Proc Am Soc Clin Oncol 1999;18:485a.
- Oka M, Fukuda M, Kuba M, et al. Phase I study of irinotecan and cisplatin
with concurrent split-course radiotherapy in limited-disease small-cell lung
cancer. Eur J Cancer 2002;38:1998-2004.
- Gray JR, Hainsworth JD, Burris HA, et al. Paclitaxel, carboplatin, and
topotecan in the treatment of small cell lung cancer: A phase II trial of
the Minnie Pearl Cancer Research Network. Proc Am Soc Clin Oncol
2000;19:494a.
- Gray JR, Hainsworth JD, Burris HA, et al. Paclitaxel/carboplatin/topotecan
with concurrent high-dose radiation therapy (RT) for the treatment
of limited stage small cell lung cancer (SCLC): a Minnie Pearl Cancer
Research Network phase II trial. Proc Am Soc Clin Oncol 2002;21:1931a.
- van de Pol M, van Oosterhout AG, Wilmink JT, et al. MRI in detection of
brain metastases at initial staging of small-cell lung cancer. Neuroradiology
1996;38:207-10.
- Seute T, Leffers P, ten Velde GP, et al. Neurologic disorders in 432 consecutive
patients with small cell lung carcinoma. Cancer 2004;100:801-06.
- Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier
to chemotherapy in metastatic brain tumors: Establishing a treatment
paradigm. J Clin Oncol 2007;25:2306-12.
- Korfel A, Oehm C, von Pawel J, et al. Response to topotecan of
symptomatic brain metastases of small-cell lung cancer also after whole-brain
irrediation. A multicentre phase II study. Eur J Cancer 2002;38:2724-9.
- Chou RH, Chen AY, Lau D. Promising role of irinotecan for the treatment
of brain metastases. J Clin Neurosci 2005;12:242-45.
- Chen G, Huynh M, Chen A, et al. Chemotherapy for brain metastases in
small-cell lung cancer. Clin Lung Cancer 2008;9:35-8.
Cite this article as: Chen AY, Chen PM, Chen YJ. DNA
topoisomerase I drugs and radiotherapy for lung cancer.
J Thorac Dis 2012;4(4):390-397. doi: 10.3978/
j.issn.2072-1439.2012.07.12
|