
Patients with asthma or chronic obstructive pulmonary disease (COPD) can generate sufficient inspiratory flows via Easyhaler® dry powder inhaler: a pooled analysis of two randomized controlled trials
Introduction
Inhaled medications are the mainstay of pharmacological treatment for asthma and chronic obstructive pulmonary disease (COPD) (1,2). Inhalation is the preferred route of administration because it enables delivery of the drug directly to the lung. Furthermore, inhaled drugs can achieve a rapid clinical effect at lower doses than those used for orally or parentally administered therapies, thereby reducing the risk of systemic side effects (3-5).
Dry powder inhalers (DPIs) are commonly used in patients with asthma and COPD (4). These devices require de-agglomeration of the powder formulation by inspiratory airflow to respirable particles (6); hence, their performance is dependent on both the patient’s inspiratory effort and the internal resistance to airflow inside the inhalation channel of the device (4). A high airflow resistance reduces the velocity of the aerosol particles within the respiratory tract, thereby increasing penetration in the lungs (7). To ensure that drug delivery is accurate and consistent, and to achieve a beneficial clinical effect, the peak inspiratory flow (PIF) with medium-to-high and high resistance DPIs, in general, should be 30 L/min or higher (6,8). While many patients are able to generate sufficient inspiratory flow rates for effective drug delivery using a DPI (9,10), the need for deep, forceful inhalation may present a challenge for some patients, such as young children and those with severe airflow limitation (4,11).
The fixed-dose combinations of salmeterol–fluticasone propionate and budesonide-formoterol have been developed to be dispensed with the Easyhaler® device-metered, multi-dose DPI (EH; Orion Corporation, Orion Pharma, Espoo, Finland), a medium-to-high resistance inhaler, for the treatment of patients with asthma and COPD (1,12,13). Data from two randomized, multicenter, crossover trials contributed to regulatory approval of these products (5,14). These trials demonstrated that patients with asthma and COPD can achieve sufficient inspiratory flows to enable the delivery of a consistent drug dose via the EH (5,14,15). Jõgi et al. evaluated inspiratory flow parameters of salmeterol-fluticasone propionate EH in subgroups of patients of varying ages with asthma and patients with COPD, and reported mean PIF rates through the EH ranging from 54 to 76 L/min, and 67 L/min, in these respective conditions (14). In another study of a similar design, PIF rates through budesonide-formoterol EH were 64 and 56 L/min in patients with asthma or COPD, respectively, and 61 L/min in a subgroup of children with asthma (5). In both studies, similar flow rate dependence for drug delivery was demonstrated for the EH, compared with Seretide Diskus (DI; GlaxoSmithKline, Brentford, UK) and Symbicort Turbuhaler (TH; AstraZeneca, Södertälje, Sweden), respectively (5,14).
However, factors that could potentially affect the patients’ ability for sufficient inhalation through DPIs were not thoroughly investigated in these studies. These factors include age and other anthropometric dimensions, gender, disease, and severity of lung function reduction. Therefore, the present post-hoc analysis of pooled EH data from the two pre-registration studies of salmeterol-fluticasone propionate EH and budesonide-formoterol EH was conducted to further evaluate whether patients with asthma (including children, adolescents adults, and the elderly) or COPD (all ages) can achieve sufficient inspiratory flows for effective use of these fixed-dose combination DPIs, and which factors affect the inspiratory flows they achieve (15). We present the following article in accordance with the STROBE Statement reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2112).
Methods
Study design
Two randomized, multicenter, crossover, open-label studies were carried out in Estonia, Finland, and the UK [ClinicalTrials.gov identifiers NCT01424137 (14) (https://doi.org/10.1089/jamp.2018.1463) and NCT00984906 (5) (https://doi.org/10.1089/jamp.2013.1099)], with all study procedures performed at a single visit. The primary objective of the studies was to characterize inspiratory flow and volume parameters across the EH, DI, and TH inhalers in patients with asthma (including children, adolescents, and adults) and/or COPD. Two types of EH inhaler were included, because at the time of the studies both devices were still under consideration as options for EH combination products: Type A is the inhaler currently used for all marketed EH combination products and Type B was an alternative design under testing. Patients were randomly allocated to 1 of 4 possible inhaler sequences involving the EH (both types) and either DI (NCT01424137) or TH (NCT00984906) inhalers: (I) TH/DI-EH Type A-EH Type B; (II) TH/DI-EH Type B-EH Type A; (III) EH Type A-EH Type B-TH/DI; or (IV) EH Type B-EH Type A-TH/DI. The inhalers either contained placebo inhalation powder (NCT01424137) or were empty (NCT00984906). On the morning of the study day, patients withheld use of their usual inhaled medication to mimic the clinical situation of inhaler use. Flow-volume spirometry was performed according to the American Thoracic Society/European Respiratory Society guidelines (16), including the recording of native PIF. Patients received standard training on use of the inhalers (according to instructions in the respective patient information leaflets). After practicing, the PIF rate through the inhaler connected to a pneumotachograph was recorded as previously described (17,18), using a SpiroMaster MX spirometer (Medikro Oy, Kuopio, Finland) before crossing over to the second and third devices in sequence. For most patients, inspiratory flow parameters were measured in a standing position, but sitting was also permitted, if necessary (e.g., for patients using wheelchairs). Three inspiratory flow curves were recorded and the best measurement (i.e., the curve with the highest PIF rate) was analyzed. Forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF), and native PIF rate were determined from spirometry.
For the purpose of this manuscript, further methodology and results are focused on the pooled analyses of EH Type A data, because it was the inhaler chosen for the salmeterol-fluticasone propionate EH and budesonide-formoterol EH products. No comparisons between EH and DI or TH are described.
Study population
Patients with a documented diagnosis of asthma and/or COPD across a range of disease severities were enrolled. Exclusion criteria were the presence of any severe chronic respiratory disease other than asthma or COPD, acute respiratory infection, or any medical condition that in the opinion of the investigator would have endangered the patient if they participated in the study (e.g., contraindications to spirometry), concurrent participation in a clinical drug study, inability to perform repeated spirometric measurements (NCT00984906 only), and lactose intolerance or severe milk allergy (the placebo preparations contained lactose; NCT01424137 only).
Study endpoints
The primary endpoint was PIF rate through the EH. The inspiratory flow values are also presented as pressure drops using device specific resistance values in the conversion. Secondary endpoints included inspiratory volume (measured at the same time as the PIF rate) and Pearson’s correlation analysis between PIF rate or inspiratory volume through the EH and age, weight, height, and various lung function parameters.
Statistical analyses
Both studies were exploratory and observational; therefore, no statistical hypotheses were specified, and evaluations were based mainly on descriptive statistics. Due to the nature of the studies, sample size was not based on any formal power calculations. Only data for patients ≥6 years of age were taken into account, because that is the age limit for prescribing the EH. Pearson correlations between PIF rate as well as inspiratory volume through the EH and lung function parameters [FEV1, FVC, native PIF, forced inspiratory vital capacity (FIVC) and PEF], age, weight, and height were evaluated by comparing the Pearson’s correlation coefficients (r). As the pressure drop only describes the same phenomenon in different units, the statistical analyses were not repeated for pressure drop. All analyses were performed in the total pooled study population, stratified according to the following three subgroups: patients with asthma 6–17 years, patients with asthma ≥18 years of age, and patients with COPD. All data were analyzed descriptively using Statistical Analysis Software (SAS)® for Windows version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Ethical conduct of the studies
The study protocols were reviewed by the ethics committees of each study center. Studies were conducted according to the principles of the Declaration of Helsinki (19), and written informed consent was obtained from participants prior to enrollment. All participants (and/or their parents) provided written informed consent prior to any study-specific procedures; prior to this, both patients and parents were given comprehensive verbal and written information regarding the objectives and procedures of the study, any possible risks and benefits involved, and their right to withdraw from the study at any time.
Results
Patients
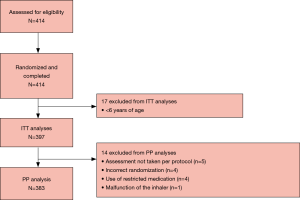
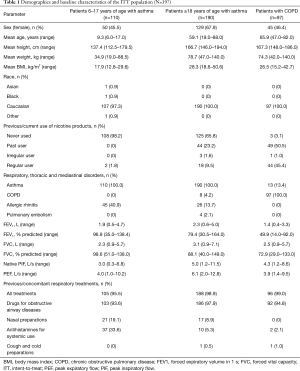
In total, 414 patients were assessed for eligibility, and all entered and completed the study (Figure 1). The intent-to-treat (ITT) population comprised 397 patients and 383 patients were included in the per-protocol (PP) population. The reasons for exclusion from the PP population were assessment not taken PP (n=5), incorrect randomization (n=4), use of restricted medication (n=4), and inhaler malfunction (n=1) (Figure 1). Demographics and baseline characteristics of the total pooled study population, including the subgroups of patients with asthma, are presented in Table 1. In the ITT population, 27.7% of patients were <18 years of age and 56.4% were female; among patients with asthma, 36.7% were <18 years of age and 59.6% were female. In total 21 patients (5.3%) had both asthma and COPD, and 71 (23.7%) asthma patients also had allergic rhinitis (Table 1). As expected, patients with COPD had poorer lung function parameters than their counterparts in the asthma cohorts.
Full table
Primary endpoint: PIF
The mean PIF (standard deviation) values through the EH in patients <18 and ≥18 years of age with asthma and in those with COPD, were similar: 61.4 (11.5), 69.7 (13.5), and 61.9 (13.2) L/min, respectively (Figure 2). These flow rates correspond to pressure drops of 5.05 (1.80), 6.52 (2.34) and 5.19 (2.07) kPa, respectively. In all subgroups females had lower PIFs than males; in patients with asthma <18 years 60.4 (11.2) vs. 62.2 (11.7) L/min, in adult patients with asthma 67.0 (12.8) vs. 75.5 (13.3) L/min and in patients with COPD 57.6 (11.9) vs. 65.7 (13.2) L/min. Of the 383 patients in the PP population, 380 (99.2%) were able to generate a PIF rate through the EH of ≥30 L/min, which is required to enable consistent dose delivery from the DPI. The three patients who did not achieve a PIF rate ≥30 L/min comprised one patient from each disease cohort (patients <18 and ≥18 years of age with asthma and those with COPD).

Secondary endpoints
Inspiratory volume
The inspiratory volume [L (range)] measured through the inhaler was 1.6 (0.6–3.8), 2.1 (0.3–4.3), and 1.8 (0.6–3.9) in patients <18 years and ≥18 years of age with asthma, and those with COPD, respectively.
Relationship of PIF with baseline parameters
In the PP population, there was a moderate direct association between age and PIF in younger patients (<18 years) with asthma, but this was inverse and less apparent in adult patients with asthma and/or those with COPD (r=0.301, P=0.0021 vs. r=–0.212, P=0.0037 and r=–0.246, P=0.0156 for patients <18 years and ≥18 years of age with asthma and patients with COPD, respectively) (Table 2). Height and weight were also moderately correlated with PIF. Stronger associations with PIF were observed for some lung function parameters, particularly native PIF (r=0.440 and r=0.662; both P<0.0001 in patients ≥18 years of age with asthma and patients with COPD, respectively) and FIVC (r=0.499–0.632, increasing with age in patients with asthma and highest in patients with COPD; P<0.0001 in all subgroups) (Table 2). The degree of reduction in ventilatory function, expressed as FEV1% predicted, was weakly associated with PIF in patients with COPD, but not in either of the asthma patient subgroups.

Full table
Relationship of inspiratory volume with baseline parameters
In general, correlations between inspiratory volume via inhaler with baseline parameters were stronger than those for PIF. The strongest association with inspiratory volume was seen for FVC in all subgroups (r=0.804, r=0.668 and r=0.723 in patients <18 years with asthma, ≥18 years of age with asthma and patients with COPD, respectively; P<0.0001 for all) (Table 3). In patients <18 years with asthma age, height, weight and FEV1 were at least moderately correlated with inspiratory volume (r higher than 0.6, P<0.0001 for all).

Full table
Discussion
This post-hoc analysis of pooled data from the inspiratory flow studies of salmeterol–fluticasone propionate EH and budesonide-formoterol EH demonstrated that patients with asthma or COPD can achieve sufficient PIF rates for effective use of the EH. The mean pressure drops are well above the 4 kPa defined both in US and European pharmacopeia method (20-22). Although the pharmacopeia methods were defined for quality control purposes (e.g., at batch release), it also highlights that the patients can operate EH in the appropriate pressure range. Over 99% of patients, irrespective of age or baseline lung function, were able to generate PIF rates ≥30 L/min, which is the minimum rate required by most medium-to-high resistance devices to achieve delivery and deposition of aerosolized drug deep into the lungs (6). Inhalers with higher internal resistance, such as the EH, require lower PIF rates for the same given pressure gradient than those with lower internal resistance (23), meaning they may be more suitable for patients with limited lung function. In clinical practice, most patients appear able to use a medium-to-high resistance DPI effectively, even during exacerbations (24). The difference in minimal and optimal PIF rates for the TH and DI reflects the wide variation in drug delivery and deposition at lower PIF rates with these DPIs; this is not seen with the EH, which demonstrates consistent drug delivery, irrespective of the inspiratory effort of the user (23). Some patients, such as young children and those with severe airflow limitation, e.g., due to COPD, may find deep, forceful inhalation challenging, and there is a concern that these patients may not be able to generate sufficient inspiratory flow to use their inhaler effectively (4). In such cases, the EH may represent a suitable choice of DPI to ensure effective treatment and management of asthma and COPD exacerbations.
In the present study, PIF was correlated with age, height, and weight in younger patients with asthma, but these correlations were weaker in adult patients with asthma or COPD. Previous studies have shown associations between younger/advanced age (17), shorter stature (25), and female sex (17,26), with reduced PIF rate in patients with COPD, and younger age with reduced PIF rate in patients with asthma (27). These associations may be mainly explained by variations in respiratory muscle function. In our patient population, strong correlations between PIF rate and most lung parameters were not observed, although notably, higher associations were reported for native PIF and FIVC than other parameters, particularly in patients with COPD, and this warrants further investigation. Overall, this mirrors the findings of other studies that evaluated associations with various patient characteristics, including absolute FEV1 and FVC (23,26). Although FEV1% predicted and PIF have not been correlated in a broad population with varying disease severity and lung function, a significant correlation has been noted in patients with severe COPD (defined as FEV1 <30% predicted) using the Ellipta DPI (r=0.73; P<0.0001) (28), suggesting a consistent reduction in PIF rate exists where there is severe airflow obstruction (23). This is in agreement with our findings, which showed only a weak association between FEV1% predicted and PIF with EH in patients with COPD, but not in patients in either of the asthma subgroups.
It is relatively rare for patients not to achieve sufficient inspiratory volume. In our study patients with asthma <18 years had the lowest inhaled volume in average. In these patients age, height, weight and FEV1 at least moderately correlated with inspiratory volume.
In the present study, participants received inhaler training prior to DPI use. The data reported support the generally accepted view that inhaler training is beneficial for increasing the proportion of patients who achieve adequate inhalation flow rates (29,30), with some studies showing that the combination of comprehensive patient counselling and enhanced training can produce up to a 30% improvement in PIF rate (27).
The strength of this study is in the high number of patients with asthma and COPD with wide age range and representing all disease severities. Even though the data set is a combination from two studies the measurements were carried out with the same equipment and instructions. In addition, although there are many studies with flow measurements through different inhalers (or through In-Check Dial device mimicking the resistance of inhalers), only a fraction of them have dealt with correlations between these parameters and baseline spirometric data, demographic or anthropometric data (31-33).
Conclusions
In this large pooled analysis of two randomized, multicenter, crossover, open-label studies including 397 patients, almost all patients with asthma and/or COPD were able to inhale through the EH with an adequate PIF rate and achieve sufficient pressure drop, irrespective of age or severity of airway obstruction. This highlights that patients with asthma and/or COPD can achieve inspiratory flows via the EH DPI that are sufficient for its effective use.
Acknowledgments
Editorial assistance in the preparation of this article was provided by David Griffiths, PhD, of Bioscript Medical and funded by Orion Corporation, Orion Pharma.
Funding: The studies reported were funded by Orion Corporation, Orion Pharma.
Footnote
Reporting Checklist: The authors have completed the STROBE checklist. Available at http://dx.doi.org/10.21037/jtd-20-2112
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2112
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-2112
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2112). LPM reports personal fees from Orion Pharma, AstraZeneca and Chiesi, all outside the submitted work. ASP reports personal fees from Orion Pharma, outside of the submitted work. VV, SL and MV are employees of Orion Corporation, Orion Pharma. RJ reports personal fees from Orion Pharma (outside of the submitted work), Novartis, Boehringer and GSK outside the submitted work.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and was conducted in compliance with the International Conference on Harmonization on Good Clinical Practice. The protocols were approved by the independent ethics committees of the participating centres and registered under the NCT01424137; NCT009849061 identifier at the clinicaltrials.gov website. Written informed consent was required from all patients prior to enrolment. All patients provided written, informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lavorini F. Easyhaler®: an overview of an inhaler device for day-to-day use in patients with asthma and chronic obstructive pulmonary disease. Drugs Context 2019;8:212596. [Crossref] [PubMed]
- Rogliani P, Calzetta L, Coppola A, et al. Optimizing drug delivery in COPD: The role of inhaler devices. Respir Med 2017;124:6-14. [Crossref] [PubMed]
- Borghardt JM, Kloft C, Sharma A. Inhaled therapy in respiratory disease: the complex interplay of pulmonary kinetic processes. Can Respir J 2018;2018:2732017. [Crossref] [PubMed]
- Lavorini F, Pistolesi M, Usmani OS. Recent advances in capsule-based dry powder inhaler technology. Multidiscip Respir Med 2017;12:11. [Crossref] [PubMed]
- Malmberg LP, Everard ML, Haikarainen J, et al. Evaluation of in vitro and in vivo flow rate dependency of budesonide/formoterol Easyhaler®. J Aerosol Med Pulm Drug Deliv 2014;27:329-40. [Crossref] [PubMed]
- Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 2011;37:1308-31. [Crossref] [PubMed]
- Frijlink HW, De Boer AH. Dry powder inhalers for pulmonary drug delivery. Expert Opin Drug Deliv 2004;1:67-86. [Crossref] [PubMed]
- Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients' inhalation maneuvers. Respir Med 2016;118:65-75. [Crossref] [PubMed]
- Borgström L, Asking L, Thorsson L. Idealhalers or realhalers? A comparison of Diskus and Turbuhaler. Int J Clin Pract 2005;59:1488-95. [Crossref] [PubMed]
- Selroos O, Borgström L, Ingelf J. Performance of Turbuhaler® in patients with acute airway obstruction and COPD, and in children with asthma: understanding the clinical importance of adequate peak inspiratory flow, high lung deposition, and low in vivo dose variability. Treat Respir Med 2006;5:305-15. [Crossref] [PubMed]
- Clark AR, Weers JG, Dhand R. The Confusing World of Dry Powder Inhalers: It Is All About Inspiratory Pressures, Not Inspiratory Flow Rates. J Aerosol Med Pulm Drug Deliv 2020;33:1-11. [Crossref] [PubMed]
- Kirjavainen M, Mattila L, Vahteristo M, et al. Pharmacokinetics of Salmeterol and Fluticasone Propionate Delivered in Combination via Easyhaler and Diskus Dry Powder Inhalers in Healthy Subjects. J Aerosol Med Pulm Drug Deliv 2018;31:290-7. [Crossref] [PubMed]
- Lähelmä S, Sairanen U, Haikarainen J, et al. Equivalent Lung Dose and Systemic Exposure of Budesonide/Formoterol Combination via Easyhaler and Turbuhaler. J Aerosol Med Pulm Drug Deliv 2015;28:462-73. [Crossref] [PubMed]
- Jõgi R, Lähelmä S, Vahteristo M, et al. In vitro flow rate dependency of delivered dose and fine particle dose of salmeterol/fluticasone propionate Easyhaler and Seretide Diskus with patient flow rates collected in a randomized controlled trial. J Aerosol Med Pulm Drug Deliv 2019;32:88-98. [Crossref] [PubMed]
- Malmberg LP, Pelkonen A, Lähelmä S, et al. Patients with asthma and patients with COPD can generate sufficient inspiratory flows via Easyhaler® dry powder inhaler. British Thoracic Society Winter Meeting; London, UK5–7 December 2018.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Malmberg LP, Rytilä P, Happonen P, et al. Inspiratory flows through dry powder inhaler in chronic obstructive pulmonary disease: age and gender rather than severity matters. Int J Chron Obstruct Pulmon Dis 2010;5:257-62. [Crossref] [PubMed]
- Malmström K, Sorva R, Silvasti M. Application and efficacy of the multi-dose powder inhaler, Easyhaler, in children with asthma. Pediatr Allergy Immunol 1999;10:66-70. [Crossref] [PubMed]
- Association. WM. WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- Inhalation and Nasal Drug Products: Aerosols, Sprays, and Powders - Performance Quality Tests [Internet]. 2020 [cited 24th Mar 2020]. Available online: https://online.uspnf.com/uspnf/document/1_GUID-FA5F788A-4449-4F16-8435-9B8D5EECB5C9_4_en-US
- European Pharmacopoeia. Preparations for inhalation: aerodynamic assessment of fine particles. [Internet]. Council of Europe, Strasbourg, France. 2008. Available online: https://pharmeuropa.edqm.eu/home
- European Pharmacopoeia. Preparations for Inhalation [Internet]. Council of Europe, Strasbourg, France. 2018. Available online: https://pharmeuropa.edqm.eu/home
- Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv 2017;30:381-7. [Crossref] [PubMed]
- Brown PH, Ning AC, Greening AP, et al. Peak inspiratory flow through Turbuhaler in acute asthma. Eur Respir J 1995;8:1940-1. [Crossref] [PubMed]
- Duarte AG, Tung L, Zhang W, et al. Spirometry Measurement of Peak Inspiratory Flow Identifies Suboptimal Use of Dry Powder Inhalers in Ambulatory Patients with COPD. Chronic Obstr Pulm Dis 2019;6:246-55. [Crossref] [PubMed]
- Ghosh S, Pleasants RA, Ohar JA, et al. Prevalence and factors associated with suboptimal peak inspiratory flow rates in COPD. Int J Chron Obstruct Pulmon Dis 2019;14:585-95. [Crossref] [PubMed]
- Azouz W, Chetcuti P, Hosker H, et al. Inhalation characteristics of asthma patients, COPD patients and healthy volunteers with the Spiromax® and Turbuhaler® devices: a randomised, cross-over study. BMC Pulm Med 2015;15:47. [Crossref] [PubMed]
- Prime D, de Backer W, Hamilton M, et al. Effect of disease severity in asthma and chronic obstructive pulmonary disease on inhaler-specific inhalation profiles through the ELLIPTA® dry powder inhaler. J Aerosol Med Pulm Drug Deliv 2015;28:486-97. [Crossref] [PubMed]
- Hawksworth GM, James L, Chrystyn H. Characterization of the inspiratory manoeuvre when asthmatics inhale through a Turbohaler pre- and post-counselling in a community pharmacy. Respir Med 2000;94:501-4. [Crossref] [PubMed]
- Nsour WM, Alldred A, Corrado J, et al. Measurement of peak inhalation rates with an in-check meter to identify an elderly patient's ability to use a turbuhaler. Respir Med 2001;95:965-8. [Crossref] [PubMed]
- Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J 2008;31:78-83. [Crossref] [PubMed]
- Kawamatawong T, Khiawwan S, Pornsuriyasak P. Peak inspiratory flow rate measurement by using In-Check DIAL for the different inhaler devices in elderly with obstructive airway diseases. J Asthma Allergy 2017;10:17-21. [Crossref] [PubMed]
- Farkas Á, Szipocs A, Horvath A, et al. Establishment of relationships between native and inhalation device specific spirometric parameters as a step towards patient tailored inhalation device selection. Respir Med 2019;154:133-40. [Crossref] [PubMed]


