Correlation of fluid balance and postoperative pulmonary complications in patients after esophagectomy for cancer
Introduction
Postoperative pulmonary complications have been reported in 17.7% to 38.0% of patients who underwent esophagectomy for cancer (1-6) and have been associated with adverse short term outcomes and decreased long term survival (1). Therefore, it is important for clinicians to make treatment plans to reduce the incidence of postoperative pulmonary complications of patients after esophagectomy.
Several risk factors have been reported to be associated with postoperative pulmonary complications after esophagectomy for cancer, including increased age, decreased forced expiratory volume at 1 second of predicted (FEV1%), decreased diffusion capacity of the lung for carbon monoxide of predicted, poor performance status, salvage esophagectomy after definitive chemoradiotherapy, and intraoperative fluid balance (2-3,6,7). There were many studies of fluid loading on the intensive care unit (ICU) and major surgery (8-10). However, few studies focused on the effect of fluid loading on pulmonary complications after esophagectomy (11,12). And no consistent conclusions have been drawn on the amount of positive fluid balance in which day in predicting the occurrence of pulmonary complications for patients after esophagectomy (11,12). Therefore, it is our aim to share our experiences of association between fluid balance and pulmonary complications after esophagectomy in a high volume hospital.
Materials and methods
Data of patients who admitted to ICU after esophagectomy at Cancer Hospital of Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC) between September 2008 and October 2010 were retrospectively collected and reviewed. Exclusion criteria were patients received exploratory thoracotomy, those who had induction therapy, patients who had incomplete data, patients who had a poor pulmonary function test result, patients who developed anastomotic leaks, and patients admitted to ICU for mechanical ventilation ≤24 h. Poor pulmonary function tests were defined as FEV1% ≤70%. Institutional review board of CAMS approved the study and patients’ consents were waived owning to the observational nature of this study.
Preoperative staging was carried out according to the guidelines of Department of Thoracic Surgery of Cancer Hospital of CAMS and PUMC, which includes whole blood test, biochemical test, chest CT, abdomen ultrasound, barium contrast study, endoscopy, pulmonary function tests, and electrocardiogram. Preoperative physiologic assessments were based on age, results of pulmonary function tests including forced expiratory volume at 1 second (FEV1), FEV1%, and diffusing capacity of the lung for carbon monoxide of predicted (DLCO%). Patients were advised to stop smoking 1 week before surgery. Patients received antibiotic therapy for at least 3-5 days if evidence of pulmonary infection exists, until symptoms relieved and imaging of the chest showing disappearance of inflammation.
General anesthesia with one-lung ventilation was performed for all patients during the operation. Patients received left transthoracic, Ivor-Lewis, or Mckeown approach based on the location of the lesion. No patients with minimally invasive esophagectomy were admitted to ICU although minimally invasive esophagectomy was started in 2009 in our hospital (13). Perioperative fluid balance was calculated from intraoperative period to postoperative 3 days or until patients discharged from ICU, that is to subtract fluid loss including intraoperative blood loss, urine output, volume of drainage from total fluid administered. As for postoperative fluid management, simple goal directed fluid therapy was used in this ICU, and the following four goals were to be considered: level of blood pressure (BP), center venous pressure, urine output and circulation of skin. First, keep BP at normal level, including keep BP at 100-110/60-70 mmHg for patients with no history of hypertension and at preoperative level for patients with history of hypertension. Second, keep CVP level at 8-12 cmH2O in our practices which resembles conservative therapy in ARDS clinical trials network (14). Third, adjust fluid volume according to whether there is edema of skin. Finally, keep urine output at >0.5 mL/kg/h. To achieve these goals, diuretic agents, fluid supplementation or vasopressors are used to keep these four variables in the ranges mentioned above. In patients who returned to surgical ward directly after operation, fluid management was carried out according to the individual judgement of volume status of patients by attending surgeons. Postoperative respiratory tract management included chest physiotherapy and early ambulation. And patient-controlled analgesia was given to every patient to control postoperative pain.
Postoperative pulmonary complications were defined as respiratory insufficiency in need of intubation, and pneumonia as documented by fever, elevated white blood cell count, and pulmonary infiltrate requiring antibiotic therapy. Pathological staging was performed using the American Joint Committee on Cancer (AJCC) Cancer Staging Handbook (7th edition) (15). Simplified acute physiology score (SAPS 3) was calculated using data within 1 h of ICU admission, which reflected severity of the patients (16).
Statistical analyses were carried out using SPSS software for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation and compared respectively using Student’s t-test. Categorical variables were reported as absolute numbers (frequency percentages) and analyzed using χ2 test. Univariable and multivariable regression analysis were used to define the predictors of postoperative pulmonary complications. The area under the receiver operating characteristic curve (AUROC) was used to evaluate the ability of the model to discriminate between patients who developed postoperative pulmonary complications or not (discrimination). A two-tailed P value <0.05 was considered statistically significant.
Results
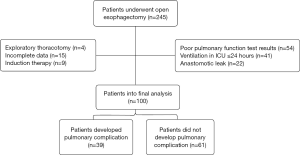
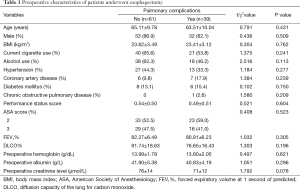
A total of 245 patients were admitted to ICU during the study period. Of which, 4 patients experienced exploratory thoracotomy, 15 patients had incomplete data, 9 patients received induction therapy, 54 patients had poor pulmonary function test results, 22 patients developed anastomotic leak, and 41 patients were ventilated ≤24 h. The rest 100 patients were enrolled into the final analysis (Figure 1). Preoperative characteristics of 100 patients are listed in Table 1. Overall, 39 patients developed postoperative pulmonary complications (39.0%) and hospital death was observed in 3 patients (3.0%). No significant differences were found on the preoperative comorbidities including a history of hypertension, coronary heart disease, diabetic mellitus and chronic obstructive pulmonary disease. And there were no significant differences on preoperative pulmonary function test results and preoperative blood test level including hemoglobin and albumin (Table 1).
Full table
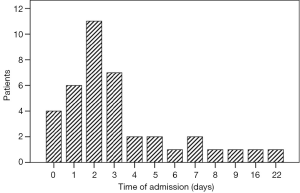
Fifty patients admitted to ICU from operative room because of comorbidities (n=45) and intraoperative arrhythmias (n=5). The other 50 patients admitted to ICU from ward because of major pulmonary complications (14 patients with pneumonias requiring antibiotic therapy and 25 patients with respiratory insufficiency in need of intubation and ventilation), postoperative arrhythmias (n=9), and high BP (n=2). Thirty nine patients admitted to ICU from ward between 1 and 22 days postoperatively, with a median time of 2 days, and 72% of all 39 patients developed pulmonary complications in 3 days postoperatively (Figure 2). Three patients died in hospital. Among 3 patients, 2 died of pulmonary infections, 1 patient died of severe acute respiratory distress syndrome.
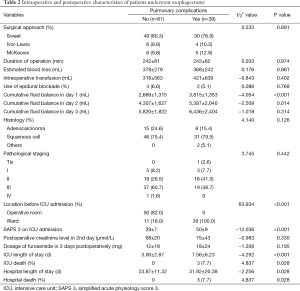
Univariable analysis showed that patients who developed postoperative pulmonary complications had more cumulative fluid balance in day 1 and day 2 (2,669±1,315 vs. 3,815±1,353 mL, P<0.001; and 4,307±1,627 vs. 5,397±2,040 mL, P=0.014) respectively, compared with patients who did not have postoperative pulmonary complications (Table 2). There were no significant differences in the perioperative creatinine level, approach of operation, duration of operation, estimated blood loss, intraoperative transfusion, use of epidural blockage, postoperative dosage of furosemide in 3 days postoperative and cumulative fluid balance in day 3. Patients who developed pulmonary complications were more severe as reflected by simplified acute physiologic score 3, and had increased ICU death, increased hospital death, prolonged ICU length of stay and hospital length of stay compared with patients who did not develop pulmonary complications (Table 2).
Full table
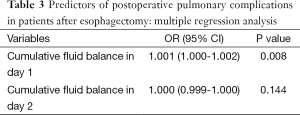
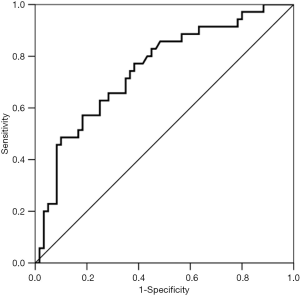
Multivariable regression analysis demonstrated that only cumulative fluid balance in day 1 (P=0.008; OR =1.001; 95% CI, 1.000-1.002) was independent risk factor for postoperative pulmonary complications (Table 3). While cumulative fluid balance in day 2 was not. AUROC of the cumulative fluid balance in postoperative day 1 was 0.749±0.052 (P<0.001, 95% CI, 0.646-0.851) in predicting the occurrence of postoperative major pulmonary complications. The cutoff value for the cumulative fluid balance in day 1 was 2,643 mL, with a sensitivity of 0.77 and specificity of 0.62 (Figure 3).
Full table
Discussion
In this study, we found that excess fluid administration in postoperative day 1 was associated with increased rate of pulmonary complications in patients after esophagectomy.
In 2008, Wei et al. demonstrated that the cumulative fluid balance from the intraoperative period to postoperative day 2 is a good predictor of surgical outcomes in patients after esophagectomy, whether for heart morbidities or pulmonary morbidities (11). They believed that stress responses increased the levels of neuroendocrine hormones, which contribute to increased capillary permeability and fluid conservation. However, they also argued that postoperative complications may also contribute to excessive fluid balance as it is difficult for clinicians to distinguish whether the fluid balance excess is a cause or a result of postoperative complications. In our study, we exclude the patients who developed complications such as anastomotic leak, which might develop septic shock and receive large volume fluid resuscitation, thereby confound the cause or result of excessive fluid balance as in Wei et al.’s study (11). Therefore, we concluded that excessive fluid infusion did increase the risk of postoperative pulmonary complications in patients after esophagectomy. In our study, cumulative fluid balance was +4.3 L on day 2 and +5.8 L on day 3. According to our experiences, most patients who underwent esophagectomy had low blood volume before surgery because of dysphagia, although preoperative blood hemoglobin and albumin level were in the normal range. Therefore, most patients received a lot of fluid postoperatively.
The mechanism of fluid balance on the development of pulmonary complications was multifactorial. First, one-lung ventilation anesthesia and pain postoperatively has been demonstrated to be risk factors for pulmonary complications (17,18). Second, excessive fluid volume and the resulting increased pulmonary capillary hydrostatic pressure and/or an increase in capillary permeability damage the endothelial glycocalyx layer (EGL), which may contribute to the development of acute lung injury (10,19). In the current study, all patients received one-lung ventilation intraoperatively and patient-controlled analgesia postoperatively. And after multivariable regressive analysis, cumulative fluid balance in postoperative day 1 was demonstrated to be the only risk factor of for the development to major pulmonary complications, which corroborate the above hypothesis of the relationship between excessive fluid volume and the development of pulmonary complications.
Casado found that fluid administration intraoperatively and postoperatively was a contributing factor for the development of respiratory complications after esophageal surgery (12). They concluded that the amount of fluid to be administered to avoid respiratory complications is 8,300 mL intraoperatively and for the next 5 postoperative days. However, in our study, 39 patients admitted to ICU from ward between 1 and 22 days postoperatively, with a median time of 2 days, and 72% of all 39 patients developed pulmonary complications in 3 days postoperatively. On the other hand, early detection positive fluid balance is critical for clinicians to reduce the rate of pulmonary complications in patients after esophagectomy. As excessive fluid administration is reversible by restricting the volume administration and giving the diuretics, therefore, the results of our study that cumulative fluid balance in day 1 was an independent risk factor for postoperative pulmonary complications provided more useful information for clinicians including surgeons and intensivists to pay more attentions on the fluid balance in the early period postoperatively. And early detection and timely management may decrease the incidence of postoperative pulmonary complications in patients after esophagectomy.
In this study, simple goal directed fluid therapy was used in ICU and traditional fluid therapy determined by attending surgeon was used in the Department of Thoracic Surgery. About 78% patients (39/50) who received traditional fluid strategy in the surgical ward developed pulmonary complications, which is significantly higher than zero in the ICU. Different fluid strategy may account for the reason. In acute lung injury patients, the conservative strategy of fluid management has been demonstrated to be superior to the liberal strategy, as reflected by shortened duration of mechanical ventilation (14). Conservative strategy has also been found to promote faster recovery after elective colorectal resection compared with the liberal strategy (20). However, excessive fluid restriction increased the level of hypovolemia, leading to reduced central venous oxygen saturation (ScvO2) and thereby increased incidence of postoperative complications (21). Therefore, the concept of goal-directed therapy has been proposed to improve perioperative outcomes. Recently, Corcoran et al. studied 23 RCTs and demonstrated that patients receiving liberal fluid therapy had a higher risk of pneumonia compared with patients receiving goal-directed therapy [risk ratio (RR) =2.2] (22). However, no comparisons between goal-directed therapy and restrictive fluid therapy were made in their study. Further studies are needed to compare the effectiveness between goal-directed therapy and restrictive fluid therapy.
Brodner et al. introduced a multi-modal approach including thoracic epidural analgesia, early tracheal extubation, and forced mobilization in order to mobilize these patients as soon as possible to prevent pulmonary complications (23). In our study, 50 patients who discharged to the ward received this multi-modal approach and 50 patients did not receive this multi-modal approach and admitted to ICU and ventilated overnight because of low pulmonary function. However, in our hospital, not all patients who underwent esophagectomy were ventilated postoperatively. In fact, almost 1,500 esophagectomy were performed in our hospital annually and less than 50 patients admitted to ICU directly from operation room and were ventilated, other patients were discharged to ward after direct extubation. Therefore, with the development of surgical technique such as minimal invasive esophagectomy, anaesthetic technique such as intraoperative lung protective ventilation, less patients who underwent esophagectomy will received ventilation post-esophagectomy. Under these circumstances, fluid administration becomes more and more important for both surgeons and intensivists.
Our study had several limitations. Firstly, only 100 patients included in this study and retrospective nature of this study may hamper the conclusion of this study. Secondly, the results of this study were from a single cancer center. However, patients were managed over two years in a high volume center, which made the results credible. Thirdly, we excluded patients who were ventilated 24 h or less which may limit the generalisability of this study. However, as we mentioned above, less than 50 patients of 1,500 patients who underwent esophagectomy annually admitted to ICU directly from operation room and were ventilated, other patients were discharged to ward after direct extubation. Therefore, excluding patients who were ventilated 24 h or less after esophagectomy will not influence the result of our study.
In conclusion, positive fluid balance in postoperative day 1 was associated with increased rate of postoperative pulmonary complications in patients after esophagectomy for cancer. Further studies are needed to validate the results of this study.
Acknowledgements
This study was carried out in the Department of Intensive Care Unit, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kinugasa S, Tachibana M, Yoshimura H, et al. Postoperative pulmonary complications are associated with worse short- and long-term outcomes after extended esophagectomy. J Surg Oncol 2004;88:71-7. [PubMed]
- Law S, Wong KH, Kwok KF, et al. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg 2004;240:791-800. [PubMed]
- Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg 2011;91:1494-1500; discussion 1500-1. [PubMed]
- D’Annoville T, D’Journo XB, Trousse D, et al. Respiratory complications after oesophagectomy for cancer do not affect disease-free survival. Eur J Cardiothorac Surg 2012;41:e66-73; discussion e73.
- Molena D, Mungo B, Stem M, et al. Outcomes of esophagectomy for esophageal achalasia in the United States. J Gastrointest Surg 2014;18:310-7. [PubMed]
- Yoshida N, Watanabe M, Baba Y, et al. Risk factors for pulmonary complications after esophagectomy for esophageal cancer. Surg Today 2014;44:526-32. [PubMed]
- Kita T, Mammoto T, Kishi Y. Fluid management and postoperative respiratory disturbances in patients with transthoracic esophagectomy for carcinoma. J Clin Anesth 2002;14:252-6. [PubMed]
- Barmparas G, Liou D, Lee D, et al. Impact of positive fluid balance on critically ill surgical patients: a prospective observational study. J Crit Care 2014;29:936-41. [PubMed]
- Lee J, de Louw E, Niemi M, et al. Association between fluid balance and survival in critically ill patients. J Intern Med 2015;277:468-77. [PubMed]
- Chau EH, Slinger P. Perioperative fluid management for pulmonary resection surgery and esophagectomy. Semin Cardiothorac Vasc Anesth 2014;18:36-44. [PubMed]
- Wei S, Tian J, Song X, et al. Association of perioperative fluid balance and adverse surgical outcomes in esophageal cancer and esophagogastric junction cancer. Ann Thorac Surg 2008;86:266-72. [PubMed]
- Casado D, López F, Martí R. Perioperative fluid management and major respiratory complications in patients undergoing esophagectomy. Dis Esophagus 2010;23:523-8. [PubMed]
- Mu J, Yuan Z, Zhang B, et al. Comparative study of minimally invasive versus open esophagectomy for esophageal cancer in a single cancer center. Chin Med J (Engl) 2014;127:747-52. [PubMed]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564-75.
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 2005;31:1345-55. [PubMed]
- Chandrashekar MV, Irving M, Wayman J, et al. Immediate extubation and epidural analgesia allow safe management in a high-dependency unit after two-stage oesophagectomy. Results of eight years of experience in a specialized upper gastrointestinal unit in a district general hospital. Br J Anaesth 2003;90:474-9. [PubMed]
- Cense HA, Lagarde SM, de Jong K, et al. Association of no epidural analgesia with postoperative morbidity and mortality after transthoracic esophageal cancer resection. J Am Coll Surg 2006;202:395-400. [PubMed]
- Assaad S, Popescu W, Perrino A. Fluid management in thoracic surgery. Curr Opin Anaesthesiol 2013;26:31-9. [PubMed]
- Brandstrup B, Tønnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003;238:641-8. [PubMed]
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: A prospective randomized trial. Arch Surg 2010;145:1193-200. [PubMed]
- Corcoran T, Rhodes JE, Clarke S, et al. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg 2012;114:640-51. [PubMed]
- Brodner G, Pogatzki E, Van Aken H, et al. A multimodal approach to control postoperative pathophysiology and rehabilitation in patients undergoing abdominothoracic esophagectomy. Anesth Analg 1998;86:228-34. [PubMed]