The past, current and future of diagnosis and management of pleural disease
Introduction
Pleural effusions affect approximately 1.5 million patients per year in the United States and remain one of the most commonly encountered entities by the chest physician (1,2). The diagnostic approach to pleural effusions has historically involved thoracentesis and pleural fluid analysis utilizing Light’s criteria, followed by increasingly invasive procedures such as closed and/or visualized pleural biopsy. These procedures remain mainstays in the evaluation of the pleural space however in recent years new imaging modalities as well as minimally invasive approaches to old techniques have added themselves to the clinicians’ armamentarium in the diagnosis pleural disease. On the other hand, the therapeutic management of pleural disease had seen little change in the last 100 years until the recent introduction of new high value techniques in the care of pleural infection, malignancy and persistent air leaks. In this review will address our past and current practices in the diagnosis and treatment of pleural disease, highlighting recent advances and potential future innovations.
Thoracic imaging in pleural disease
Chest radiography
The investigation and management of pleural disease has historically relied heavily on thoracic imaging. Chest radiographs (CXR) particularly the erect posterior-anterior view are frequently the first imaging used to assess the pleural space. Lateral and decubitus radiographs are now performed less often to the increasing availability and superiority of point-of-care ultrasound (US) and computed tomography (CT) (3-5).
Ultrasonography
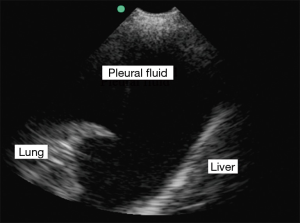
Thoracic US has become increasingly the modality of choice for the evaluation of pleural disease. Once adequate education has been achieved US has proven itself to be portable, easy to use, within improved patient safety and increased diagnostic accuracy (3). The data now strongly supports an improvement in patient safety with the use of US when performing thoracentesis and should be considered standard of care (6-8). Ultrasonography has also shown itself to be superior to portable CXR in the detection of pleural effusions, estimation of volume, prediction of the fluid characteristics and in guided intervention (4,9-11) (Figure 1). When compared with the historic practice of CXR and physical examination prior to thoracentesis, US has been shown to be far superior in identifying an appropriate needle entrance point, even in the hands of ‘experts’ in diagnosis and management of chest disease (12). Several studies also suggest a lack of inferiority of US to CT when assessing the pleural space and that the real-time imaging may add additional information not available on CT (13).
Computed and positron-emission tomography
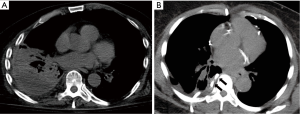
The use of thoracic CT in the diagnosis and management of pleural disease has become increasingly common place. Use of delayed intravenous contrast is often used to optimize pleural soft-tissue enhancement (3,14,15). High-resolution CT scanning can be used in the detection of pleural plaques and should be performed with 1 mm and high spatial resolution reconstruction algorithm. One important characteristic of the thoracic CT is its ability to not only evaluate the pleural space but to also reveal underlying lung parenchymal, mediastinal and/or hilar disease. Thoracic CT has also been adapted to procedural performance. CT-guided placement of chest drains, thoracentesis and in the biopsy of the parietal pleura, have been well documented with excellent diagnostic yields and safety (16-19) (Figure 2). Positron-emission tomography in combination with CT has been shown to be useful in patients with focal or generalized pleural thickening suspicious for malignancy (15). The major limitation of CT and positron emission tomography (PET) imaging is its lack of portability to the patient’s bedside, with the inherent difficulty of performing repeated examinations and associated radiation exposure.
Magnetic resonance imaging
Historically thought to have a limited role, magnetic resonance imaging (MRI) has begun to find its place in the diagnosis and management of pleural disease. New advances in MRI technology has allowed for improved image quality of the thorax and specifically the pleural space (20). Improvements in MRI signal and interpretation has led to the ability to differentiate between such entities as transudates, exudates, hematoma, malignant pleural implants and chest wall invasion. Differentiation between causes of exudative effusions such as parapneumonic vs. malignant effusion vs. empyema remains challenging. Recent studies into functional MRI have led to the suggestion that it can be used as a modality to assess for treatment response of malignant pleural disease (21).
Pleural fluid analysis
Fluid analysis has long been the initial step in evaluation of pleural disease presenting as an idiopathic effusion. First reported in 1972, Light’s criteria gave clinicians an easy set of criteria by which to classify pleural effusions as either transudate or exudate (22). Light’s criteria has since been criticized for over classifying exudates and the reliance on serum measurements. This has led to a series of proposed modifications that include measurement of pleural fluid cholesterol and albumin. In 1987, Hamm et al. were the first to present the measurement of cholesterol as a marker for differentiation between transudative and exudative pleural effusion (23). This has been followed by multiple studies including a meta-analysis confirming the utility of cholesterol as an aid in pleural fluid characterization (24-26). The serum to protein albumin gradient has also show itself to be useful in the characterization of pleural fluid (27). This measure appears to be quite helpful in properly evaluating those patients with a clinical picture consistent with a transudative process but found to have an exudate when their pleural fluid is analyzed, the so called “false-exudate”, commonly seen in patients with congestive heart failure who have received diuretic therapy (28). The amino-terminal fragment of pro-brain natriuretic peptide (NT-proBNP) has also been evaluated as an adjunct test to aid in the differentiation of pleural effusions when a “false-exudate” is suspected. Porcel et al., evaluated 93 patients with pleural effusion and found that using a cut-off of 1,500 pg/mL, the receiver operating characteristic curve for the diagnosis of heart failure effusion was 0.931 for NT-proBNP in the pleural fluid. This makes NT-proBNP a very useful marker when faced with the diagnostic dilemma of the “false-exudate”, however, it tends to correlate extremely well with serum NT-BNP, and thus serum NT-BNP can be used to make the diagnosis (29,30).
Parapneumonic effusions and empyema
Despite advances in the treatment of pneumonia, the incidence of pleural disease related to infection continues rise (31-33). Of patients presenting with a parapneumonic effusion, 10% will develop an empyema and 20% will require surgical intervention (34). Typical first steps when being presented with a pneumonia and suspected parapneumonic pleural process are sampling of the effusion to further characterize it as simple, complex or empyema and the administration of broad spectrum antibiotics tailored to the local microbiome. This is often followed by placement of a chest drain in an attempt to fully drain the pleural space and to achieve source control. Controversy exists around the management of pleural infection when failure to fully drain the pleural space occurs after chest drain placement. Surgical intervention in the setting of pleural infection that has failed medical therapy has been recommended by the current guidelines and has been considered first line therapy in the United States (34). In the MIST2 trial, Rahman et al., evaluated the effect of tPA and DNase alone and in combination on drainage of pleural infection by way of radiographic improvement. They were able to show a significant improvement in the CXR of those patients who received combination versus single agent or placebo fibrinolysis (35). Further study of this subject is necessary to better understand this technique’s effect on mortality, morbidity and length of stay. In addition, a group of patients in the MIST2 trial went on to surgery after fibrinolytic administration, an issue that warrants further study in an effort to better risk stratify patients prior to intervention.
The first attempt at pre-interventional risk stratification was recently published by Rahman et al., who developed a scoring system (RAPID) to aimed to identifying those patients at high risk of death from pleural infection (36). White et al., retrospectively evaluated the RAPID score of 187 patients in an effort at assess increased risk of mortality at 3 months, 1, 3 and 5 years respectively. They reported a significant in increase mortality among the medium- and high-risk groups at all of the measured time points when compared to the low-risk group (37).
Medical thoracoscopy
Single port medical thoracoscopy has become increasingly common place in its use by pulmonologists in diagnosis and treatment of pleural disease. Medical thoracoscopy has gone from supplanting closed pleural biopsy in the diagnosis of malignant and infectious diseases (38,39) to including such interventions as talc poudrage in the setting of pneumothorax and malignant pleural effusion and in the treatment of complex parapneumonic effusion and empyema. In addition, semi-rigid thoracoscopes have been shown to have similar diagnostic yields to rigid thoracoscopes despite smaller biopsy size (40,41).
Empyema
In the United States and UK, invasive surgical intervention for complex parapneumonic effusion and empyema not responding to medical therapy remains the provenance of the thoracic surgeon. Three non-randomized, non-comparator case series have recently reported high ‘success rates’ and low or no complications in patients undergoing medical thoracoscopy for pleural infection (42). These case series present data that indicates a potential role for medical thoracoscopy in the treatment these diseases, however prospective randomized data is required before the applicability of medical thoracoscopy to pleural infections can be recommended.
Pneumothorax
The treatment of primary spontaneous pneumothorax via semi-rigid or rigid medical thoracoscopy with talc poudrage has gained increasing acceptance a therapeutic modality in those patients with persistent air leaks or recurrent pneumothorax, with a reported 93% durable success rate and potential cost savings when compared with standard chest tube drainage (43,44). However, surgical intervention utilizing single lung ventilation and mechanical vs. talc pleurodesis remains the “gold standard” for treatment of this disease process.
In an elegant study, Noppen et al. performed a comparison of white light medical thoracoscopy with thoracoscopy utilizing fluorescein-enhanced autofluorescence to assess pleural integrity in patients with primary spontaneous pneumothorax (PSP) compared to controls. In those patients with primary spontaneous pneumothorax, autofluorescence showed abnormalities of the pleura in those areas that appeared normal under white light, suggesting that significant parenchymal abnormalities outside of readily visible blebs and bullae were present and likely responsible for the recurrent nature of the disease (45). As such, evidence suggests significantly lower recurrence rates when pleurodesis is performed as opposed to bullectomy alone.
Persistent air leaks
Bronchopleural fistula (BPF)/alveolar-pleural fistula or persistent air leak (PAL) is a cause of significant morbidity and mortality after parenchymal lung resection, spontaneous pneumothorax, and/or chronic suppurative lung disease. The incidence of BPF post lobectomy or pneumonectomy has been reported to be as high as 4.5%, with an associated mortality of up to 27%. In those patients with BPF, up to 90% require a repeat surgical procedure (46). PAL can lead to prolonged hospitalization and complications that result in increased health care costs (47).
Many non-surgical methods have been attempted to treat post-resection PAL with varying degrees of success. The most conservative treatment is prolonged chest tube placement with either a pleural drainage system or Heimlich valve. Pleural-based methods include pleurodesis via mechanical, chemical, and autologous means (48-50). These methods of pleurodesis have not been shown to have reliable efficacy, require that the lung has fully re-expanded and carry with them their own associated risks (51). Bronchoscopic attempts to treat PAL have include vascular coils, fibrin or tissue glue, spigots, stents, gel foam, silver nitrate, and autologous endobronchial blood patch (52,53). While these methods have all shown promise, each has its limitations, and none have shown significant efficacy to replace surgical intervention in the treatment of post-resection PAL.
Endobronchial valves
Two one-way endobronchial airway valves (EBV) placed for the purpose of promoting segmental lung atelectasis currently exist. The Emphasys® Zephyr® Endobronchial valve (ZEBV, Pulmonx Inc., Neuchatel Switzerland) is a 2nd generation device that consists of a silicone “duck bill” attached to a nitinol skeleton and is reported to have a lower airway resistance profile than previous models (Figure 3). The Intrabronchial Valve, (IBV, Spiration Inc., Redmond, WA, USA) uses a polymer membrane suspended from a nitinol framework shaped like an umbrella to act as a one-way valve (Figure 4).
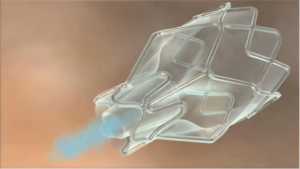
EBV were originally developed for the purpose of performing bronchoscopic lung volume reduction (BLVR) to treat patients with heterogeneous emphysema and hyperinflation. Though neither of the airway valve technologies has received FDA approval for BLVR they have been used successfully in the closure of persistent post-surgical BPF. The IBV was approved by the FDA in 2006 for use under the Humanitarian Device Exemption program for patients with PAL after lung parenchymal resection (54).
EBV can be placed via flexible bronchoscopy under general or moderate anesthesia. A pleural drainage system must be in place to evaluate changes in air leak during the procedure. Prior to EBV placement, balloon occlusion is used to identify the bronchus or bronchi involved. The EBV is then deployed into the airway through the working channel of the bronchoscope and the patient’s chest drain is observed for cessation or decrease in the PAL. The EBV are subsequently removed 4-6 weeks after placement and resolution of the air leak in order to minimize the risk of infection, granulation, migration, or expectoration of the valve (55).
The use of endobronchial valves for treatment of PAL was first described in three case reports in 2005 and 2006 (56-58). In all of these cases EBV were used to treat persistent post-operative air leaks, which had been present in one case for over 6 years, and resulted in resolution of the PAL. Since these first reports, a number of case series and reports have been published describing the use of EBV to treat PAL in post-operative patients and those with spontaneous pneumothorax with low complication rates (59-67). These case series suggest that EBV placement is a safe and effective therapy for BPF and PAL after lung resection, spontaneous pneumothorax, and suppurative lung or pleural infection.
The use of EBV in the critical care setting has also been reported, including in patients requiring mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and as a bridge to lung transplantation (68-70).
Development and regular use of less invasive strategies for the management of BPF/PAL in patients has the potential to improve patient morbidity. Studies to date, however, are limited by small sample size and lack of a comparison group. Prospective randomized controlled trials are needed to assess the efficacy of EBV placement in the treatment of BPF/PAL.
Management of malignant pleural effusions
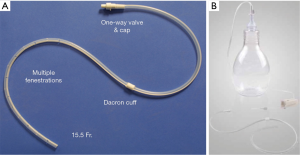
Recent advances in malignant pleural disease has been primarily focused on the use of tunneled pleural catheters (TPCs) in the management of dyspnea, hospital length of stay, quality of life and cost effectiveness of the procedure. The catheter is a 15 French fenestrated drain that is inserted into the pleural space and then tunneled subcutaneously to reduce the risk of infectious complications (Figure 5). The majority of these procedures can be done in an outpatient setting. Since their introduction, TPCs have become increasingly ubiquitous with the palliative management of malignant pleural disease. This technology continues to be rigorously studied as providers search for less invasive management options than classic surgical intervention. Shown to be safe and effective in the treatment of malignant pleural effusions (MPE) (71,72), the study of TPCs has turned toward comparisons with invasive surgical techniques and chemical pleurodesis. In a study aimed at developing a method of “rapid” pleurodesis for the management of MPE, thoracoscopy, talc pleurodesis and TPC placement were performed simultaneously followed by aggressive outpatient TPC drainage (Figure 6). Compared with TPC spontaneous pleurodesis rates of 40-60% and a historical length of stay (LOS) of 5 days following talc poudrage, pleurodesis and length of stay were found to be 92% and a median of 1.79 days in the “rapid pleurodesis” cohort respectively. This pilot study was not powered nor randomized to assess a statistical difference between modalities and prospective randomized trials are needed (73). Hunt et al., retrospectively reviewed 109 patients who underwent thoracoscopy and talc poudrage vs. TPC placement for symptomatic MPE. Patients undergoing TPC placement had significantly shorter LOS and lower rates of reintervention (74). In a propensity matched retrospective study comparing TPC to thoracoscopy and talc poudrage, Freeman et al., reported that patients undergoing TPC placement for MPE had significantly shorter LOS, interval to systemic therapy and lower rates of operative morbidity (75). Two recent prospective studies of MPE treatment comparing TPC and talc pleurodesis, via slurry or poudrage, reported significantly shorter LOS, improvements in dyspnea, quality of life (QOL) and fewer episodes of pleural reintervention (76,77). Puri et al., published a cost effectiveness study of the treatment of MPE that used decision analysis to compare repeated thoracentesis, TPC, bedside talc slurry and talc poudrage. They reported that when comparing pleurodesis strategies for patients with short expected survival time (3 months) TPC was the preferred treatment strategy due to decreased cost and improved efficacy. They went on to report that for patients with longer expected survival times (12 months) that bedside pleurodesis was more cost effective (78). Sabur and colleagues published the first study specifically examining the effect of TPCs on QOL in patients with MPE. They reported that TPCs were associated with significant improvements in global health status, QOL and dyspnea at 2 weeks follow up from pre-procedure baseline. Due to the death of 45% of the patients enrolled, measures at 14 weeks were improved but did not reach statistical significance (79).
Conclusions
Pleural effusions remain one of the most frequently encountered disease processes by the thoracic medicine practitioner. Though this disease process continues to challenge physicians, advances have been made in the diagnosis and management of pleural effusion. Previous methods of evaluating the etiology of pleural disease remain useful and have been modified improving their sensitivity and specificity. Imaging of the pleural space has continued to advance diagnostic capability and advances in the treatment and diagnosis of pleural infection have been profound. With these advances come the need for improved risk stratification for patients with pleural infection. Malignant pleural effusion management has seen great strides with the development of TPC allowing patients to palliate themselves at home and avoid further invasive procedures. These improvements in the diagnosis and treatment of pleural effusions have set the stage for future advances in our understanding of pleural pathophysiology and patient care.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Light RW. Pleural Diseases. 4th ed. Wilkins LW, editor. Philadelphia, 2001.
- Maskell N; British Thoracic Society Pleural Disease Guideline Group. British Thoracic Society Pleural Disease Guidelines--2010 update. Thorax 2010;65:667-9. [PubMed]
- Ayres J, Gleeson F. Imaging of the pleura. Semin Respir Crit Care Med 2010;31:674-88. [PubMed]
- Kohan JM, Poe RH, Israel RH, et al. Value of chest ultrasonography versus decubitus roentgenography for thoracentesis. Am Rev Respir Dis 1986;133:1124-6. [PubMed]
- Eibenberger KL, Dock WI, Ammann ME, et al. Quantification of pleural effusions: sonography versus radiography. Radiology 1994;191:681-4. [PubMed]
- Colt HG, Brewer N, Barbur E. Evaluation of patient-related and procedure-related factors contributing to pneumothorax following thoracentesis. Chest 1999;116:134-8. [PubMed]
- Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med 1990;150:873-7. [PubMed]
- Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010;170:332-9. [PubMed]
- Yang PC, Luh KT, Chang DB, et al. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol 1992;159:29-33. [PubMed]
- Yu CJ, Yang PC, Chang DB, et al. Diagnostic and therapeutic use of chest sonography: value in critically ill patients. AJR Am J Roentgenol 1992;159:695-701. [PubMed]
- Gryminski J, Krakówka P, Lypacewicz G. The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest 1976;70:33-7. [PubMed]
- Diacon AH, Brutsche MH, Solèr M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 2003;123:436-41. [PubMed]
- Yu CJ, Yang PC, Wu HD, et al. Ultrasound study in unilateral hemithorax opacification. Image comparison with computed tomography. Am Rev Respir Dis 1993;147:430-4. [PubMed]
- Tsujimoto N, Saraya T, Light RW, et al. A Simple Method for Differentiating Complicated Parapneumonic Effusion/Empyema from Parapneumonic Effusion Using the Split Pleura Sign and the Amount of Pleural Effusion on Thoracic CT. PLoS One 2015;10:e0130141. [PubMed]
- Evans AL, Gleeson FV. Radiology in pleural disease: state of the art. Respirology 2004;9:300-12. [PubMed]
- Raptopoulos V, Davis LM, Lee G, et al. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol 1991;156:917-20. [PubMed]
- Hyde J, Sykes T, Graham T. Reducing morbidity from chest drains. BMJ 1997;314:914-5. [PubMed]
- Patz EF Jr, Goodman PC, Erasmus JJ. Percutaneous drainage of pleural collections. J Thorac Imaging 1998;13:83-92. [PubMed]
- Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003;361:1326-30. [PubMed]
- Coolen J, Verschakelen J, De Wever W. MRI of pleural diseases. Curr Opin Pulm Med 2015;21:399-406. [PubMed]
- Bonomo L, Feragalli B, Sacco R, et al. Malignant pleural disease. Eur J Radiol 2000;34:98-118. [PubMed]
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13. [PubMed]
- Hamm H, Brohan U, Bohmer R, et al. Cholesterol in pleural effusions. A diagnostic aid. Chest 1987;92:296-302. [PubMed]
- Valdés L, Pose A, Suàrez J, et al. Cholesterol: a useful parameter for distinguishing between pleural exudates and transudates. Chest 1991;99:1097-102. [PubMed]
- Gil Suay V, Martínez Moragón E, Cases Viedma E, et al. Pleural cholesterol in differentiating transudates and exudates. A prospective study of 232 cases. Respiration 1995;62:57-63. [PubMed]
- Shen Y, Zhu H, Wan C, et al. Can cholesterol be used to distinguish pleural exudates from transudates? evidence from a bivariate meta-analysis. BMC Pulm Med 2014;14:61. [PubMed]
- Roth BJ, O'Meara TF, Cragun WH. The serum-effusion albumin gradient in the evaluation of pleural effusions. Chest 1990;98:546-9. [PubMed]
- Bielsa S, Porcel JM, Castellote J, et al. Solving the Light's criteria misclassification rate of cardiac and hepatic transudates. Respirology 2012;17:721-6. [PubMed]
- Porcel JM, Chorda J, Cao G, et al. Comparing serum and pleural fluid pro-brain natriuretic peptide (NT-proBNP) levels with pleural-to-serum albumin gradient for the identification of cardiac effusions misclassified by Light's criteria. Respirology 2007;12:654-9. [PubMed]
- Kolditz M, Halank M, Schiemanck CS, et al. High diagnostic accuracy of NT-proBNP for cardiac origin of pleural effusions. Eur Respir J 2006;28:144-50. [PubMed]
- Farjah F, Symons RG, Krishnadasan B, et al. Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg 2007;133:346-51. [PubMed]
- Fletcher MA, Schmitt HJ, Syrochkina M, et al. Pneumococcal empyema and complicated pneumonias: global trends in incidence, prevalence, and serotype epidemiology. Eur J Clin Microbiol Infect Dis 2014;33:879-910. [PubMed]
- Grijalva CG, Zhu Y, Nuorti JP, et al. Emergence of parapneumonic empyema in the USA. Thorax 2011;66:663-8. [PubMed]
- Corcoran JP, Wrightson JM, Belcher E, et al. Pleural infection: past, present, and future directions. Lancet Respir Med 2015;3:563-77. [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [PubMed]
- Rahman NM, Kahan BC, Miller RF, et al. A clinical score (RAPID) to identify those at risk for poor outcome at presentation in patients with pleural infection. Chest 2014;145:848-55. [PubMed]
- White HD, Henry C, Stock EM, et al. Predicting Long-Term Outcomes in Pleural Infections. RAPID Score for Risk Stratification. Ann Am Thorac Soc 2015;12:1310-6. [PubMed]
- Emad A, Rezaian GR. Diagnostic value of closed percutaneous pleural biopsy vs pleuroscopy in suspected malignant pleural effusion or tuberculous pleurisy in a region with a high incidence of tuberculosis: a comparative, age-dependent study. Respir Med 1998;92:488-92. [PubMed]
- Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest 2007;131:261-74. [PubMed]
- Khan MA, Ambalavanan S, Thomson D, et al. A comparison of the diagnostic yield of rigid and semirigid thoracoscopes. J Bronchology Interv Pulmonol 2012;19:98-101. [PubMed]
- Dhooria S, Singh N, Aggarwal AN, et al. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care 2014;59:756-64. [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii54-60. [PubMed]
- Tschopp JM, Brutsche M, Frey JG. Treatment of complicated spontaneous pneumothorax by simple talc pleurodesis under thoracoscopy and local anaesthesia. Thorax 1997;52:329-32. [PubMed]
- Tschopp JM, Boutin C, Astoul P, et al. Talcage by medical thoracoscopy for primary spontaneous pneumothorax is more cost-effective than drainage: a randomised study. Eur Respir J 2002;20:1003-9. [PubMed]
- Noppen M, Dekeukeleire T, Hanon S, et al. Fluorescein-enhanced autofluorescence thoracoscopy in patients with primary spontaneous pneumothorax and normal subjects. Am J Respir Crit Care Med 2006;174:26-30. [PubMed]
- Sirbu H, Busch T, Aleksic I, et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001;7:330-6. [PubMed]
- Liang S, Ivanovic J, Gilbert S, et al. Quantifying the incidence and impact of postoperative prolonged alveolar air leak after pulmonary resection. J Thorac Cardiovasc Surg 2013;145:948-54. [PubMed]
- Martínez-Escobar S, Ruiz-Bailén M, Lorente-Acosta MJ, et al. Pleurodesis using autologous blood: a new concept in the management of persistent air leak in acute respiratory distress syndrome. J Crit Care 2006;21:209-16. [PubMed]
- Oliveira FH, Cataneo DC, Ruiz RL Jr, et al. Persistent pleuropulmonary air leak treated with autologous blood: results from a university hospital and review of literature. Respiration 2010;79:302-6. [PubMed]
- Venuta F, Rendina EA, De Giacomo T, et al. Postoperative strategies to treat permanent air leaks. Thorac Surg Clin 2010;20:391-7. [PubMed]
- Kelly MG. Acute respiratory distress syndrome (ARDS) secondary to talc pleurodesis. Eur J Intern Med 2007;18:611. [PubMed]
- Sivrikoz CM, Kaya T, Tulay CM, et al. Effective approach for the treatment of bronchopleural fistula: application of endovascular metallic ring-shaped coil in combination with fibrin glue. Ann Thorac Surg 2007;83:2199-201. [PubMed]
- Watanabe S, Watanabe T, Urayama H. Endobronchial occlusion method of bronchopleural fistula with metallic coils and glue. Thorac Cardiovasc Surg 2003;51:106-8. [PubMed]
- Wood DE, Cerfolio RJ, Gonzalez X, et al. Bronchoscopic management of prolonged air leak. Clin Chest Med 2010;31:127-33. Table of Contents. [PubMed]
- Mahajan AK, Doeing DC, Hogarth DK. Isolation of persistent air leaks and placement of intrabronchial valves. J Thorac Cardiovasc Surg 2013;145:626-30. [PubMed]
- Snell GI, Holsworth L, Fowler S, et al. Occlusion of a broncho-cutaneous fistula with endobronchial one-way valves. Ann Thorac Surg 2005;80:1930-2. [PubMed]
- Feller-Kopman D, Bechara R, Garland R, et al. Use of a removable endobronchial valve for the treatment of bronchopleural fistula. Chest 2006;130:273-5. [PubMed]
- Mitchell KM, Boley TM, Hazelrigg SR. Endobronchial valves for treatment of bronchopleural fistula. Ann Thorac Surg 2006;81:1129-31. [PubMed]
- Gillespie CT, Sterman DH, Cerfolio RJ, et al. Endobronchial valve treatment for prolonged air leaks of the lung: a case series. Ann Thorac Surg 2011;91:270-3. [PubMed]
- El-Sameed Y, Waness A, Al Shamsi I, et al. Endobronchial valves in the management of broncho-pleural and alveolo-pleural fistulae. Lung 2012;190:347-51. [PubMed]
- Travaline JM, McKenna RJ Jr, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest 2009;136:355-60. [PubMed]
- Firlinger I, Stubenberger E, Müller MR, et al. Endoscopic one-way valve implantation in patients with prolonged air leak and the use of digital air leak monitoring. Ann Thorac Surg 2013;95:1243-9. [PubMed]
- Dooms CA, Decaluwe H, Yserbyt J, et al. Bronchial valve treatment for pulmonary air leak after anatomical lung resection for cancer. Eur Respir J 2014;43:1142-8. [PubMed]
- Yu WC, Yeung YC, Chang Y, et al. Use of endobronchial one-way valves reveals questions on etiology of spontaneous pneumothorax: report of three cases. J Cardiothorac Surg 2009;4:63. [PubMed]
- Santini M, Fiorelli A, Vicidomini G, et al. Iatrogenic air leak successfully treated by bronchoscopic placement of unidirectional endobronchial valves. Ann Thorac Surg 2010;89:2007-10. [PubMed]
- Schweigert M, Kraus D, Ficker JH, et al. Closure of persisting air leaks in patients with severe pleural empyema--use of endoscopic one-way endobronchial valve. Eur J Cardiothorac Surg 2011;39:401-3. [PubMed]
- van Zeller M, Bastos P, Fernandes G, et al. Clinical challenges of persistent pulmonary air-leaks--case report. Rev Port Pneumol 2014;20:162-6. [PubMed]
- Mahajan AK, Verhoef P, Patel SB, et al. Intrabronchial valves: a case series describing a minimally invasive approach to bronchopleural fistulas in medical intensive care unit patients. J Bronchology Interv Pulmonol 2012;19:137-41. [PubMed]
- Brichon PY, Poquet C, Arvieux C, et al. Successful treatment of a life-threatening air leakage, complicating severe abdominal sepsis, with a one-way endobronchial valve. Interact Cardiovasc Thorac Surg 2012;15:779-80. [PubMed]
- Fischer W, Feller-Kopman D, Shah A, et al. Endobronchial valve therapy for pneumothorax as a bridge to lung transplantation. J Heart Lung Transplant 2012;31:334-6. [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [PubMed]
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011;6:762-7. [PubMed]
- Reddy C, Ernst A, Lamb C, et al. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011;139:1419-23. [PubMed]
- Hunt BM, Farivar AS, Vallières E, et al. Thoracoscopic talc versus tunneled pleural catheters for palliation of malignant pleural effusions. Ann Thorac Surg 2012;94:1053-7; discussion 1057-9. [PubMed]
- Freeman RK, Ascioti AJ, Mahidhara RS. A propensity-matched comparison of pleurodesis or tunneled pleural catheter in patients undergoing diagnostic thoracoscopy for malignancy. Ann Thorac Surg 2013;96:259-63: discussion 263-4.
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [PubMed]
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012;142:394-400. [PubMed]
- Puri V, Pyrdeck TL, Crabtree TD, et al. Treatment of malignant pleural effusion: a cost-effectiveness analysis. Ann Thorac Surg 2012;94:374-9; discussion 379-80. [PubMed]
- Sabur NF, Chee A, Stather DR, et al. The impact of tunneled pleural catheters on the quality of life of patients with malignant pleural effusions. Respiration 2013;85:36-42. [PubMed]



