Spray cryotherapy (SCT): institutional evolution of techniques and clinical practice from early experience in the treatment of malignant airway disease
Introduction
Cryotherapy has been used successfully in bronchoscopy since 1981 (1). Until recently, cryotherapy was delivered in the airways via a cryoprobe with a gas cryogen (usually nitrogen or carbon dioxide) utilizing the Joule-Thompson effect to cool the probe tip and freeze the tissue on contact (2). Temperatures usually reach −40 to −90 °C. This device is relatively inexpensive and widely available. Although the cryoprobe has been shown to be an effective tool in bronchoscopy, it is limited by the requirement for direct contact with the tissue and relatively small area of treatment possible for each application (3-5). Liquid nitrogen has been used since the 1950s externally in a noncontact delivery spray bottle to skin lesions but no such device was available for endoscopic approaches (6). In 1999 endoscopic spray cryotherapy (SCT) using liquid nitrogen as the cryogen was developed for endoscopic delivery and received indications for the treatment of esophageal malignancies (7,8). This endoscopic technology delivers liquid nitrogen through a 7F disposable catheter that causes the flash freezing of tissue at −196 °C. This temperature difference is an important factor in the effect on the tissue. SCT results in a flash freeze of the tissue (drop in temp >100 degrees/min). This flash freeze differs from the slow freeze that occurs with probe cryotherapy where cell death occurs mainly from extracellular ice crystal formation vs. intracellular ice crystal formation with SCT (9). This flash freeze results in cell death with preservation of the extracellular matrix and regenerative regrowth of the tissue (10). The major difference between probe cryotherapy and SCT is the non-contact function of SCT, but with this comes a formidable challenge for management in the airways. When liquid nitrogen undergoes phase transformation to a gas in the endoluminal space there is a large volume expansion of 700× requiring that the newly formed gas be evacuated from the space to prevent barotrauma. When used in gastroenterology (GI) procedures this evacuation occurs with a combined suction catheter and passively through an additional port in the suction catheter. An additional large suction catheter in the airways is not practical and might worsen hypoxia during the procedure. Therefore to use SCT in the airway one must rely on passive venting through the airway and any conduit to the airway (11,12).
As with many GI endoscopic tools, SCT transitioned to airway use in some centers already using it in GI procedures (13). A multi-institutional retrospective registry reported the results of the use of this system in 80 patients with malignant airway tumor (MAT) (14). That registry reported overall successful intervention on MAT with a durable response, however, there was a 19% rate of complications including one cardiac arrest and 3 deaths. It is thought that these complications may be the result of insufficient venting of nitrogen gas. Another single center experience of 100 patients who had SCT applied to the digestive tract, supraglottic, or tracheobronchial tree reported only one pneumothorax after instrumentation prior to SCT to remove a foreign body (15). The reported pneumothorax spontaneously resolved.
In 2012 a new device was introduced that was Food and Drug Administration (FDA) approved for use in surgical procedures where passive venting, such as is used in bronchoscopic treatment in the airways. This device utilized an adjustable flow rate to allow for a wider margin of safety in the airways by delivering the liquid nitrogen at a slower rate. We previously reported our first case experiences with the new TruFeeze device (11). In the following report, we describe our initial SCT experience and how our current practice and conceptual application of this novel technology has evolved in our institution for treatment of MAT.
Materials and methods
We describe the early experience at Walter Reed National Military Medical Center from 2011 to 2013 using SCT in patients with malignant airway disease and the evolution of our current techniques and clinical practice patterns for SCT use in patients. In November 2013 enrollment began in a multi-institutional prospective SCT registry in which we are still enrolling which will be reported on separately. We describe the patient demographics, comorbidities, type of endobronchial disease, and procedural detail.
During this period from 2011 to 2013, we transitioned from the second-generation model of SCT, the G2 CryoSpray Ablation System (SCS System, Model CC2-NAM, CSA Medical Inc., Baltimore, MD) to the current SCT system, TruFreeze system (CSA Medical Inc., USA). Both systems deliver liquid nitrogen through a 7F disposable catheter at 25 watts of energy. The catheter is advanced through the working channel (minimum diameter 2.8 mm) of a bronchoscope. The catheter is directed at the target and the liquid nitrogen is delivered at low pressure to the tissue flash-freezing a 2–3 cm oval target at a depth of freeze of up to 5 mm depending on the flow setting, length of freeze, and tissue temperature and composition. The FDA approved the use of the G2 device in endoscopy with active decompression. All G2 use in the airway was considered an off-label use because only passive decompression could be employed. All patients at our institution were consented for this off label use of the device. In 2012, the newer TruFreeze (CSA Medical Inc., USA) model was FDA approved for general surgical use in both active and passive venting. In November of 2012, the first patient was treated using this device at Walter Reed (11). The delivery mechanism was similar, but the new device allowed for adjustable flow rates from a low flow (12.5 watts) to a normal flow (25 watts) setting. Detailed description of techniques for use of this novel device and the additional low flow settings previously published in 2013 is expanded upon and illustrated with follow data and imaging.
Results
Twenty-seven patients underwent 80 procedures (2.96 procedures/patient). Of these, 16 (59%) were male and 11 (41%) were female. The average age was 63 years with a range of 20 to 87 years old. The average Eastern Cooperative Oncology Group (ECOG) status was 1.26. Twenty-two (81%) of the patients had significant comorbidities of which the most common were hypertension, hyperlipidemia, and chronic obstructive pulmonary disease (COPD) respectively. All malignancies were advanced stage and all non-small cell lung cancer (NSCLC) were stage IV with the exception of one IIIb. The most common diagnosis was pulmonary adenocarcinoma in 12 (44%), followed by squamous cell carcinoma and small cell carcinoma with 4 (15%) patients each.
Of the 80 procedures 18 (22.5%) were done with the G2 system. The remaining 62 (77.5%) were done with the TruFreeze system. Twenty-five (31%) of the procedures were done through a rigid bronchoscope and 55 (69%) were done with an endotracheal tube (ETT) in place. All procedures were done under general anesthesia or deep sedation in the bronchoscopy suite. The location of the treatment was the right mainstem bronchus in 33 (41%), left mainstem bronchus in 30 (38%), trachea or main carina in 9 (11%), and multiple locations in 8 (3%). The average number of sprays per procedure was 3.6. The low flow setting was used in 38 (48%), high flow in 24 (30%), and a combination of low and high flow in 18 (23%) of the procedures. Other modalities were employed in 31 (39%) of procedures. Many of the procedures had more than two modalities employed. The following modalities were used: balloon bronchoplasty 12 (15%), mechanical debulking/rigid coring 13 (16%), electrocautery or argon plasma coagulation (APC) 5 (6%), laser 5 (6%), cryoprobe 5 (6%), and stent placement in 6 (8%). Additionally 45 of the 80 (56%) procedures were performed in proximity to a silicone, hybrid, or metal stent. Three complications occurred out of the 80 procedures (5%). All three were transient hypoxia lasting longer than several minutes. These patients were all discharged from the bronchoscopy recovery room to their presurgical state. Of the desaturations two occurred with the G2 system and one occurred with the TruFreeze system. In all three patients we were able to return the patient to normal oxygenation and discharge them home. After these incidents, we changed our protocol and began to monitor expired fraction of inspired oxygen (FIO2) on the ventilator and would not start subsequent sprays until the exhaled oxygen saturation matched the pre-spray baseline.
Discussion
As with most interventional pulmonary practices, we were very familiar with the utility of probe cryotherapy, as well as, the limitations of using a device that required direct contact and needed to defrost prior to movement to avoid tearing of the tissue. When our GI colleagues introduced us to SCT we realized the potential of having a non-contact cryotherapy tool for use in the airways. Unfortunately, as noted in the introduction, early adopters across the nation using SCT in the airways experienced significant complications (14). In the airways, this expansion of the liquid nitrogen into gas has two major side effects. First is the large volume of cold nitrogen gas produced immediately after the phase change from liquid to gas in a ratio of approximately 1:700. In GI use, this byproduct gas is managed by an active suction catheter placed in the stomach prior to initiation of SCT in the esophagus. This catheter has two channels. First is the active suction channel and the second is a passive venting channel that allows nitrogen gas to escape freely to the atmosphere. In the airways, active suctioning is not feasible because active suctioning of air from the lungs can reduce oxygen content available for respiration and could collapse portions of lung reducing the airway caliber available for passive venting of the newly formed nitrogen gas. Also, any additional tubing in the airway besides the bronchoscope and intubating modality (ETT or rigid barrel) ultimately decreased the available working space to treat tumor and passively vent gases. Anatomically, the airways are designed for gas exchange with cartilage rings stenting the airways open unlike the esophagus, which not only has no such supportive rings, but also has muscle layers that produce peristalsis and actively contract in response to expansion. With these factors in mind, the technique for SCT in the airways developed with the use of passive venting only. Successful passive venting depends on the area of continuous pathway from the site of nitrogen liquid to gas transformation and the atmosphere outside the patient’s body. Usually an ETT or a rigid bronchoscope ensures this pathway. Since the rigid bronchoscope cannot be compressed and when open at the proximal end allows direct access to the atmosphere with a fixed diameter tube it is presumed to be the safest mode of passive venting in SCT. The ETT is also considered a very reliable conduit for passive venting provided there is no compromise of its flexible diameter due to excessive angles, kinks, patient biting, etc. Unfortunately due to the current catheter size, a therapeutic flexible bronchoscope with a minimum inner diameter of 2.8 mm outer diameter of 5.9 to 6.3 or larger is required for use of SCT. This larger than normal diameter for the bronchoscope reduces the area available for egress of the nitrogen gas in any given size conduit rigid tube or ETT. For this reason, a larger ETT tube is considered safer. Currently, an 8.5 mm ETT or a standard adult rigid bronchoscope (usually 9 mm inner diameter or greater) is recommended for ensuring safe passive egress of the large amount of nitrogen gas produced in SCT (16).
The second risk factor in nitrogen gas formation in SCT that needs to be managed is the nitrogen gas displacement of oxygen in the lungs. Although the air we breathe at sea level is approximately 78% nitrogen and 20% oxygen, the large amount of nitrogen produced instantaneously in the lungs with the liquid to gas transformation of the nitrogen will quickly displace some of the oxygen in the lungs and reduce the concentration of oxygen and may cause transient hypoxia. The degree of nitrogen present in the patient’s lungs is dependent upon the duration of the freeze, initial oxygen concentration in the lungs, passive egress pathway and ability to ventilate before and after the spray.
Freeze duration
Prior to the release of the latest model of SCT with the TruFreeze (CSA Medical) system, there was only one flow rate for liquid nitrogen delivery through the catheter so essentially duration of the freeze was the most important variable in how much nitrogen gas formed each second. In GI use with active venting, spray duration was often 20–30 s or longer for each freeze cycle (8). Even though SCT was being delivered in the esophagus, patients often experienced transient hypoxia from the inhalation of the venting nitrogen gas. With this in mind, we realized that similar duration of freeze directly in the airways was likely to result in earlier and more significant hypoxia so for the airways and passive venting, freeze duration times were often much less. Based on reported techniques we initially started with freeze times of 5–10 s (12). If the patient had good oxygen saturations throughout the freeze we would even use 10–15 s of freezing depending on the area being treated and desired effect. As our practice evolved, we found that with longer freeze times, patients would become more hypoxic, take longer to recover back to their baseline oxygen saturation and then desaturate more quickly with subsequent freezes. To mitigate this risk, we began to note the baseline inspired and expired oxygen levels attempting to maximize both in each patient prior to initiating SCT and then waiting until the baseline maximized oxygen levels were achieved to perform the next freeze (11). We also changed our practice to limit freeze duration to 5–10 s for each cycle and just increase the number of cycles if more cryogen was needed for treatment. Over the past several years we have further refined our technique to limit each freeze to 5 s to minimize the risk of barotrauma and nitrogen displacement of oxygen during the procedure. The exception to this is with the use of the low flow setting on the TruFreeze model that was first used in November of 2012. The low flow setting delivers liquid nitrogen at half the rate of the normal flow. This flow rate produces approximately half amount of nitrogen gas per unit of time as the normal flow so we allow 10 s of freeze on this setting and assume we displace similar amounts of oxygen as we do with a 5 s freeze on the normal flow setting. Even at 10 s on the low flow rate, the risk for barotrauma from nitrogen gas formation should be less than with the normal flow for 5 s because less gas is produced per second. We also have altered our practice to begin timing of the freeze duration at the start of the first visible frost on the tissue instead of the previously reported standard starting time after 50% of the target area was frosted (14). This limits the variability in freeze duration when treating different size lesions.
Initial concentration of oxygen
Recognizing that with the transformation of liquid nitrogen to it’s gas form, a large amount of nitrogen gas will displace oxygen from the lungs, we have made it our practice to always preoxygenate the patient to the highest level that we can prior to starting SCT. This concept is used often to intubating patients to increase the oxygen reserve and washout some of the 80% nitrogen found in room air. This is also a distinguishing advantage of SCT when used as an ablative surgical tool in the airways. Most of the other tools for ablation available to bronchoscopists involve heat sources that have the inherent risk of causing an airway fire if the oxygen concentration is too high when using the device. Decreasing the oxygen concentration to less than 40% is recommended when using these thermal ablation devices but this is not always possible depending on the patient’s oxygen requirement (4). Approximately 2–3 min of ventilation with 100% oxygen routinely allowed for maximum nitrogen washout and minimization of the duration and degree of hypoxia if any following the freeze. When using an ETT, we annotate the starting inspired and expired oxygen concentration on an individual patient after the initial preoxygenation. Following each freeze cycle, if there is a desaturation, we wait until we return to baseline levels of inspired and expired oxygen. The oxygen saturation on the pulse oximeter will return to baseline much sooner than the expired FIO2 and is not a reliable marker that nitrogen has been effectively removed.
Passive venting pathway
The role of passive venting in SCT in the airways is central to safe use of the modality. As this concept is not applicable to our other commonly used tools for airway tissue ablation, our understanding of this technique has evolved over the years and a limited understanding in earlier years may have contributed to earlier reported problems with it’s transition from GI to pulmonary use. The basic principle is that the newly formed nitrogen gas needs an unobstructed path to the atmosphere that is large enough to accommodate the volume of gas formed during each freeze. With the earlier SCT device (G2), delivery of liquid nitrogen was not uniform and small boluses of liquid nitrogen would come out in spurts. This delivery could produce spikes in the volume of gas produced so we routinely used a large rigid bronchoscope disconnected from the jet ventilator and open to the room to ensure plenty of room for gas egress. With a rigid metal tube in place we could also be confident there was no reduction in diameter of the venting space from compression or kinking. With the newer TruFreeze system and advances in catheter design and system cooling, a much more uniform and even delivery of liquid nitrogen was achieved. This made calculation of the area needed to adequately passively vent newly formed gas at the normal flow rate more accurate. Recommendations for adequate passive venting when using a flexible bronchoscope with the SCT catheter within a conduit tube into the airway (i.e., ETT or rigid bronchoscope) was given as the annular area around the bronchoscope for the entire pathway to the atmosphere outside the patient. Essentially with any of the commonly used therapeutic bronchoscopes, using a 8.5 mm or greater ETT or a 9.0 mm rigid barrel will allow for adequate passive venting of the nitrogen gas formed when liquid nitrogen was delivered at the normal flow rate (16).
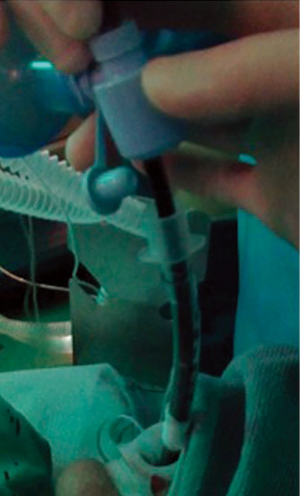
This assumes that there is no obstruction of the tube providing the annular space around the bronchoscope. It is also important to ensure that the pathway from the tube to the atmosphere is unobstructed. Our practice is to disconnect the ETT connector from the ETT (Figure 1) or to remove the cap from the rigid scope proximal end if it was being used to allow for egress to the atmosphere. We have always deflated the ETT cuff prior to SCT to allow for gas to exit around the ETT if the route is unobstructed, but this practice was started prior to the newer TruFreeze system and complying with the annular space recommendations should allow adequate passive ventilation regardless of whether or not the ETT cuff is deflated. We also have a practice of designating at least one person to monitor the visible portion of the passive venting pathway to ensure that we don’t start SCT with an obstruction and that an obstructing doesn’t occur during SCT. Even the bronchoscopist could inadvertently obstruct the egress with his hand if he were holding the scope near the ETT or rigid barrel. Usually visible sign of gas egress is seen as a white frosting mist exiting the ETT, rigid tube or mouth shortly after the liquid nitrogen begins to freeze the tissue. Some institutions have been successful using SCT with a laryngeal mask airway (LMA) or even trans nasally with moderate sedation. Egress of gas can be accomplished, but there is always a risk of laryngospasm that might impede passive venting. We currently only use a rigid bronchoscope or an ETT to ensure the vocal cords remain open through the spray. LMA has the same issue as well as possibly becoming dislodged during the procedure and allowing nitrogen gas to vent down into the stomach. We also prefer not to use LMA. Suspension laryngoscopy with paralyzed vocal cords should be sufficient to keep the vocal cords open during a spray if needed.
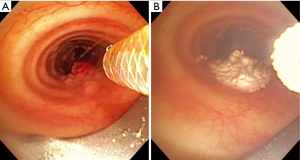
Ensuring the passive venting pathway has adequate annular space through whatever conduit into the airway is used is important, but this concept must also be applied strictly to the airway beyond the conduit. This can be challenging given the irregular shapes and degree of obstruction found in airways with tumor and stenosis. For partially obstructing tumor, special attention needs to be made to ensure that gas is not trapped distal to the obstructing lesion. The area of treatment with SCT should only include the large airways. All of our SCT applications were restricted to the trachea, left main bronchus to the left secondary carina, and right main bronchus and bronchus intermedius. Only 3% were multiple locations. We believe this reflects a selection bias where cases with multiple locations of obstructing endobronchial tumor were not offered SCT due to the complexity of ensuring adequate passive venting when treating the more distal of the multiple locations. One example would be a patient with a tracheal tumor and a more distal right main bronchus tumor that would need treatment (Figure 2A). To ensure an adequate passive venting pathway, the tracheal tumor would need to be treated (Figure 2B) and debulked and the airway secured before the more distal tumor could be treated. This concept of securing an adequate path for passive venting while proceeding more distal is essential for safe adoption of this technology.
SCT with stents
Heat therapies must be used with caution around hybrid or silicone stents due to the risk of combustion and airway fire. There is no risk of airway fire with SCT and we took advantage of this using it in 56% of our cases around stents without complication and in many cases SCT was used to maintain stent patency. We attempt to remove stents when it appears that the cause of their obstruction has resolved and the use of SCT decreases the amount of granulation tissue that can build up around the stent decreasing the difficulty of stent removal. As the cryoprobe relies on contact the use of it around stents can cause dislodgement unlike the non-contact application of SCT.
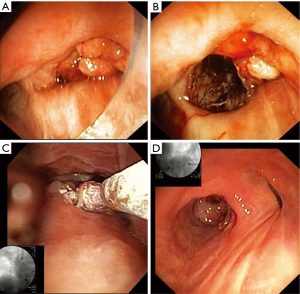
Figure 3 illustrates the use of SCT in a patient reported in our initial report of the TruFeeze use (11). Specific details of the first 7 months of treatment are described in that report, but the last 3 months of treatment with stent removal and imaging above were not available at the time of publication. In brief summary, the patient above was referred with refractory stage IV lung adenocarcinoma and presented September 2012 with a resting oxygen requirement of 2 L nasal cannula and severe dyspnea. On bronchoscopy, a large obstructing friable tumor was completely obstructing the right main bronchus (Figure 3A). A self-expanding silicone covered nitinol stent was placed to open the right lower lobe (Figure 3B), which after clearing of mucus significantly decreased the oxygen requirement. Once the airway was open, SCT was used serially to treat the tumor at the proximal end of the stent (Figure 3C). The tumor in the airway regressed but over the next several months, granulation tissue developed at the proximal stent and SCT was used to treat the granulation tissue. Eventually 10 months later in July 2013, the stent was removed and the airway mucosa had returned to a more normal state (Figure 3D). The patient passed away in March of 2014 with advanced disease metastatic to the brain but had no respiratory symptoms, did not require any supplemental oxygen and from computed tomography imaging, there was no tumor recurrence within the large airways. This technique of using SCT around stents and to treat both tumor and granulation tissue has been an important part of our practice of preserving the central airways in our advanced cancer patients.
SCT with debulking
SCT can be used like the heat ablation modalities to pre treat tumor prior to debulking. Although SCT does not vaporize or char the tissue, there is a hemostatic effect from the crystallization of the blood in the small vessels. Typically we freeze the visible tumor to a blanched color and then mechanically debulk and then repeat until we have achieved the desired effect for the airway (Figure 4). This is no different from using heat therapies to treat prior to debulking except when we reach the tissue near the base of the tumor where there is a combination of normal and malignant tissue. If vaporization or quick destruction of the tumor is needed, we have used laser or electrocautery/hot snare to remove unwanted tissue quickly and then treat the base and surrounding tissue with SCT. This transition area is ideal for SCT because although the freezing will destroy both normal and abnormal tissue, the preservation of the underlying tissue matrix with SCT promotes the regrowth of normal tissue in contrast to the scar tissue that forms after thermal ablation.

SCT without debulking
To truly take advantage of the non-contact delivery feature of SCT, we modified our practice patterns over time to partner with our oncologists to begin treatments of obstructing endobronchial tumor prior to severe obstruction (50% or greater). All of our malignant patients treated with SCT had advanced disease so our goal was palliation of dyspnea, hypoxia, hemoptysis and other conditions like post obstructive pneumonia that would worsen their quality of life or keep them from participating in clinical trials for treatment of their disease. Avoiding unnecessary debulking of tumor in the airway and allowing a slower necrosis with less disruption of the supporting tissue and vascular structures became our part of our practice pattern. Using this technique may result in additional bronchoscopies as the effects of cryotherapy are not immediate and necessitate that the patient undergo multiple invasive procedures (18). Depending on the individual patient and their comorbidities, oxygen reserve, extent and location of disease, the risk of multiple procedures performed earlier in their course of disease may very well be less risk than a single procedure when oxygen requirements are increased and airway compromise is worse. In our series the average number of procedures was 2.96 procedures per patient. In many cases SCT was used to maintain airway patency. None of the patients that died did so of a central airway obstruction. All either succumbed to progression of their malignancy outside of the trachea-bronchial tree or died of an etiology other than a malignancy.
SCT with other endobronchial tools
Other modalities were used in 39% of our cases where SCT was used. Heat therapies were often used in conjunction with SCT when there was some need to ablate or vaporize a tumor quickly. Each tool we have in bronchoscopy has advantages and disadvantages. Understanding the unique qualities of SCT and how to apply them best to the individual patient is critical to successful use of the modality. Late referral may also increase the need for use of multiple modalities in a particular case. As mentioned earlier, if we were using SCT, we would treat tumor margin and base area with SCT after using the other modalities. The exception to this is if we were not confident that the airway walls were intact after the ablative therapies, debulking or stent removal/revision. Any disruption of the integrity of the trachea-bronchial wall might allow nitrogen gas to escape into the adjacent vessels resulting in nitrogen gas embolism or create pneumothorax or pneumomediastinum.
Normal flow vs. low flow setting
This feature is described in our previously published report as primarily a mode for increased safety secondary to a slower rate of gas formation (11). While this is still valid, we have since been using this mode for treatment of smaller areas where less overspray is desired. Conceptually the low flow can be thought of as selecting a smaller circle for brush size on computer paint/editing applications. The major disadvantage to low flow is the longer time from start of spray to actual freeze starting on the tissue surface. Some patients do not have enough reserve to remain apneic or off ventilator for the time needed get the catheter cooled enough to get the liquid nitrogen to the tissue still in liquid form. To minimize this time we will often partially pre-freeze the catheter outside the patient. In this cohort, we used low flow setting in 38 (48%) and a combination of low and high flow in 18 (23%).
SCT practice patterns—“staged procedures” concept
In any particular patient at a given treatment site, SCT may have variable effects on the tissue depending on tissue composition, adjacent heat sources (vessels) and freeze duration/area. Since the effects are not usually visible other than the immediate blanching of the tissue, we prefer to intentionally use the first procedure to evaluate the effects of the SCT on the individual patient with a plan to reevaluate bronchoscopically for individual effect. At this point we have the option to treat again with the same number of cycles, decrease or increase the cycles of 5-s applications during the procedure or not treat at all if not needed. We initially started evaluating 2 weeks after the SCT treatment and would remove inflammatory tissue from the treatment site. As our understanding of the healing process with SCT developed from previous studies (10,13) and our own observation, we increased the interval to the first observation to a minimum of 3–4 weeks and allowed time for the normal tissue to heal without further manipulation or disruption. As the tumor regressed and normal tissue returned, interval between procedures increased from 6 to 12 weeks and then often no further treatments were needed despite progression of disease elsewhere in the body. We also stopped treatment if any ulceration of the surrounding treatment area was noted to avoid injury to the cartilage or fistula formation. Although heat ablative tools also cause more damage to the tissues than seen during the initial application, it is often not fully appreciated. With SCT, it has to be assumed that the area treated (visibly frosted) has been affected even if an immediate response in the tissue is not seen. While this may seem like a disadvantage, the unique feature of SCT is in the fact that the tissue matrix is not disrupted allowing for regrowth of normal epithelium with minimal scarring (10). This allows for treatment of the tumor boarders or infiltrating tumor along the airway walls. This effect may be even more important in benign disease of the airways.
This staged approach is similar to the technique often used by GI for serial treatment and surveillance of Barrett’s esophagus and palliation for esophageal tumors (8). Changing our practice and referral pattern to allow for earlier evaluation of MAT, decreasing risk at each individual procedure, especially if SCT can be delivered without any direct contact to the tumor and no debulking or other intervention is required is a result of the special properties of this treatment modality.
Conclusions
SCT is a unique tool for the bronchoscopic treatment of MAT that is fundamentally different from probe cryotherapy. In contrast to probe cryotherapy, SCT is non-contact and produces flash freezing at −196 °C which preserves the extracellular matrix and promotes regenerative regrowth of normal tissue. Using SCT safely primarily depends upon understanding the concept of passive venting and applying it to each patient to manage the nitrogen gas produced in the airway during the freeze. Routine use of SCT for palliation of dyspnea/hypoxia and respiratory complications from malignancy is safe in the proximity of stents, airway tumor, and granulation tissue, bleeding tissue and in conjunction with other commonly used modalities for bronchoscopic treatment of MAT. After noting the experience in GI and other institutions along with our own early experience with SCT we have fundamentally changed our approach to endobronchial tumor management for palliation in advanced malignancies involving the central airways. Preservation of the central airways with a staged approach using SCT has become our preferred clinical practice and at least in this initial cohort of advanced stage cancer patients SCT appeared to be safe and achieved the desired outcome of minimizing dyspnea and respiratory complications in our patients. Further prospective studies are underway and more are needed to help fully understand the optimal role for SCT in malignant and benign airway disease.
Acknowledgements
Robert Browning, MD, received research/travel funding from the Geneva Foundation and Boston University.
Footnote
Conflicts of Interest: Robert Browning, MD, serves as a consultant for CSA Medical. The other authors have no conflicts of interest to declare.
Disclaimer: The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. The identification of specific products or scientific instrumentation does not constitute endorsement or implied endorsement on the part of the author, DoD, or any component agency. While we generally excise references to products, companies, manufacturers, organizations, etc. in government produced works, the abstracts produced and other similarly situated researcher presents a special circumstance when such product inclusions become an integral part of the scientific endeavor.
References
- Sanderson DR, Neel HB 3rd, Fontana RS. Bronchoscopic cryotherapy. Ann Otol Rhinol Laryngol 1981;90:354-8. [PubMed]
- Bruley ME. A study of safety and performance requirements for cryosurgical devices. Springfield (VA): FDA/BMDDP, National Technical Information Service, 1980.
- George PJ, Rudd RM. Bronchoscopic cryotherapy for advanced bronchial carcinoma. Thorax 1991;46:150. [PubMed]
- Mathur PN. Application of Laser, Electrocautery, Argon Plasma Coagulation, and Cryotherapy in Flexible Bronchoscopy. In: Wang KP, Mehta AC, Turner JF Jr, editors. Flexible Bronchoscopy. 3rd edition. Oxford: Wiley-Blackwell, 2012:201-11.
- Lee SH, Choi WJ, Sung SW, et al. Endoscopic cryotherapy of lung and bronchial tumors: a systematic review. Korean J Intern Med 2011;26:137-44. [PubMed]
- Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med 1950;72:153-5. [PubMed]
- Johnston CM, Schoenfeld LP, Mysore JV, et al. Endoscopic spray cryotherapy: a new technique for mucosal ablation in the esophagus. Gastrointest Endosc 1999;50:86-92. [PubMed]
- Johnston MH, Eastone JA, Horwhat JD, et al. Cryoablation of Barrett's esophagus: a pilot study. Gastrointest Endosc 2005;62:842-8. [PubMed]
- Yiu WK, Basco MT, Aruny JE, et al. Cryosurgery: A review. Int J Angiol 2007;16:1-6. [PubMed]
- Godwin BL, Coad JE. Healing responses following cryothermic and hyperthermic tissue ablation. Proc. SPIE 2009;7181, Energy-based Treatment of Tissue and Assessment V, 718103.
- Browning R, Parrish S, Sarkar S, et al. First report of a novel liquid nitrogen adjustable flow spray cryotherapy (SCT) device in the bronchoscopic treatment of disease of the central tracheo-bronchial airways. J Thorac Dis 2013;5:E103-6. [PubMed]
- Krimsky WS, Sarkar S, Harley D, et al. A single center experience with spray cryotherapy in the aerodigestive tract and chest. Chest 2009;136:140S.
- Krimsky WS, Broussard JN, Sarkar SA, et al. Bronchoscopic spray cryotherapy: assessment of safety and depth of airway injury. J Thorac Cardiovasc Surg 2010;139:781-2. [PubMed]
- Finley DJ, Dycoco J, Sarkar S, et al. Airway spray cryotherapy: initial outcomes from a multiinstitutional registry. Ann Thorac Surg 2012;94:199-203; discussion 203-4. [PubMed]
- Au JT, Carson J, Monette S, et al. Spray cryotherapy is effective for bronchoscopic, endoscopic and open ablation of thoracic tissues. Interact Cardiovasc Thorac Surg 2012;15:580-4. [PubMed]
- TruFreeze System, TruFreeze Spray Kit. K113021. Decision Date 02/07/2012. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn_template.cfm?id=k113021
- Browning R, Turner JF Jr, Parrish S. SCT with tumor debulking. Asvide 2015;2:158. Available online: http://www.asvide.com/articles/736
- Greenhill S. New SCT probe with adjustable flow rates now in use with bronchoscopy. Practical Reviews in Chest Medicine 2013;9.


