Effects of acute hyperglycaemia on cardiovascular homeostasis: does a spoonful of sugar make the flow-mediated dilatation go down?
Introduction
Patients with diabetes mellitus are at substantially increased risk for adverse outcomes in association with occurrence of acute coronary syndromes (1) and in the presence of atrial fibrillation (2). Although the occurrence of acute myocardial dysfunction in the presence of hyperglycaemia has been shown to be associated with poor short-term outcomes, the issue of the contribution of instantaneous (or recent) elevation of blood sugar level (BSL) to this risk remains incompletely evaluated. Currently there is only fragmentary understanding of the potential nexus between elevation of BSL and thrombotic diathesis.
A number of studies in the literature have evaluated the cardiovascular effects of transient increases in BSL, whether in normal subjects or in patients with underlying cardiometabolic disease states in virtually all cases focusing on effects on vascular reactivity, and in particular vascular endothelial function. We now examine the significance of these findings, their implications regarding nitric oxide (NO) signalling in other tissue such as platelets, and the potential mechanisms underlying these physiological changes. Finally, we review the arguments for rapid reversal of hyperglycaemia during cardiovascular crises as a form of ancillary therapeutic measure.
Impact of hyperglycaemia on the generation and signalling of NO
Acute elevation of BSL is associated with increases in oxidative stress [for review see (3)], and hence has the potential to result in disordered vascular, myocardial and platelet physiology. In practice, effects of hyperglycaemia on vascular function might theoretically involve impairment of generation of NO, for example via increased tissue concentrations of the NO synthase inhibitor asymmetric dimethylarginine (ADMA) (4) and/or via increased tissue arginase activity (5), either of which might also be associated with “uncoupling” of NO synthase. On the other hand, increased oxidative stress in association with hyperglycaemia might well contribute to “scavenging” of NO by superoxide anion (O2–) and/or partial inactivation of soluble guanylate cyclase (sGC), resulting in attenuation of tissue responses (6) to NO (see Figure 1 for schematic representation).
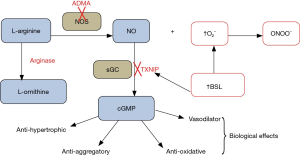
Assessment of vascular function using flow-mediated dilatation (FMD)
FMD represents one of several techniques in common clinical use which can quantitate vascular endothelial function (7), in this case via measuring post-ischemic reactive hyperaemia (largely NO-independent). Investigation of FMD physiology suggests that the hyperaemic response of the circulation to a period of relative ischemia is mediated largely by formation and release of NO (8). On the other hand, few investigations have addressed the extent to which FMD responses reflect changes in NO generation versus integrity of NO signalling: indeed it has been found that there is only a moderate correlation in individual patients between magnitude of FMD and extent of response to NO donors (9), as a probe of integrity of NO signalling pathways. Recent studies have also raised some doubts about the reproducibility of FMD data for individual subjects (10), somewhat limiting the clinical utility of this measure.
The significance of findings from Loader et al. [2015]
A recent study (11) examined the impact of acute glucose loading on FMD, utilizing a design involving meta-analysis of the published literature, focusing on 39 articles. The vast majority of these studies had utilized changes in FMD (as a “macrovascular” test of endothelial function) in healthy subjects treated with a single oral glucose load (usually of 75 grams). A minority of studies had evaluated similar changes in type 2 diabetic subjects. Few studies had evaluated “vascular smooth muscle function” simultaneously. However, as this evaluation was achieved via infusion of either sodium nitroprusside or glyceryl trinitrate (GTN) (both NO donors), the process was actually an evaluation of integrity of vascular NO signalling, rather than vascular smooth muscle function. In summary, the available data suggested a decrease in FMD of approximately 1.5% in both normal subjects and type 2 diabetics in the presence of acute hyperglycaemia. On the other hand, there was no consistent change in responses to NO donors during acute hyperglycaemia.
Superficially, this analysis argues that the adverse effects of acute hyperglycaemia on vascular function are mediated largely or entirely by decreased formation of NO. Therefore it is appropriate that we examine the known effects of acute hyperglycaemia on factors such as kinetics of ADMA and of arginases, which might represent mechanisms for decreasing NO release.
Potential mechanisms affecting NO signalling during hyperglycaemia
There is some evidence that activation of tissue arginases may be insulin-dependent. For example, Kashyap et al. (12) showed a direct correlation between extent of hyperglycaemia in diabetics and plasma arginase activity, with insulin infusion decreasing arginase activity. Ishizaka et al. (5) also showed that hyperglycaemia in rabbits was associated with enhanced arginase activity. A number of studies have also linked hyperglycaemia with increased ADMA production. For example, Mah et al. (13) showed that ADMA concentrations increase with post-prandial hyperglycaemia. Therefore the finding that FMD decreases with increasing BSL is easily explained by data of this type, although it is somewhat surprising that glucose loading in diabetics, which would be expected to more markedly increase oxidative stress, does not lead to greater changes in FMD.
The total failure of this meta-analysis to document variability in vascular responses to NO according to BSL is, however, surprising. For example, Adams et al. (9) previously documented (Figure 2) that FMD responses are directly correlated with extent of vascular response to NO donors, a finding which suggests partial commonality of controlling factors. In order to understand this more fully, it is appropriate to consider the literature related to NO responses in platelets, where influence of variable NO generation tends to be less important than integrity of signalling mechanisms.
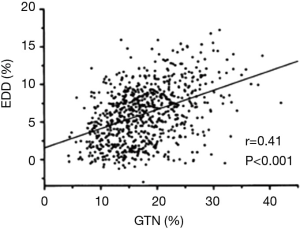
Given the known mechanistic overlap (Figure 1) and the previously demonstrated nexus between FMD and NO response (9), it is possible that the failure of some studies to document changes in vascular responses to NO donors in response to hyperglycaemia results from the common practice of utilizing drug doses which induce near-maximal responses.
Studies in platelets: impact of hyperglycaemia
The major stimulus for evaluation of the impact of changes in BSL on platelet responsiveness to NO and its determinants has been a series of clinical findings which indirectly implicate hyperglycaemia as a focus of impaired NO signalling. Hyperglycaemia represents a basis for increased mortality risk in acute myocardial infarction (14) and the results of the DIGAMI-I trial suggest that rapid reversal of hyperglycaemia by intravenously infused insulin might also reverse this risk (15).
Is there a need to reverse hyperglycaemia during cardiovascular crisis?
In 2007, Worthley et al. (16) reported that in diabetic patients with acute coronary syndromes there was an inverse relationship between instantaneous BSL and extent of inhibition of platelet aggregation by the NO donor sodium nitroprusside. This reflected primarily incremental “scavenging” of NO by O2− release. With insulin infusion leading to rapid reversal of hyperglycaemia, there was also a fall in O2− generation, together with marked improvement in NO response.
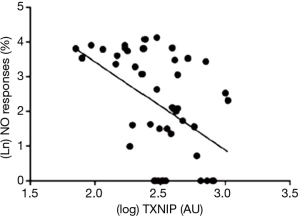
More recently, we have noted that the pro-inflammatory protein thioredoxin-interacting protein (TXNIP) appears to control platelet NO signalling under chronic conditions irrespective of hyperglycaemia: there was a reciprocal relationship between NO response and platelet TXNIP content at steady state (17) (Figure 3), while treatment with ramipril simultaneously suppressed TXNIP expression and potentiated platelet NO signalling (17,18). It would be expected that TXNIP expression would also change in response to variability in BSL: after all, there is a glucose response element on the gene coding for TXNIP expression (19). However, platelet TXNIP content did not fall significantly over 12 hours of insulin infusion in hyperglycaemic patients (20), despite restoration of NO responses, suggesting that the associated falls in O2− release were TXNIP-independent. This evidence of relatively slow changes in TXNIP expression may be relatively specific for platelets by virtue of limited DNA content. Previous studies suggest that TXNIP expression may be more rapidly adjusted in vasculature (21). It seems more likely that insulin-induced suppression of protein kinase C-dependent activation of NAD(P)H oxidase (22) may have been critical to decreases in O2− formation affecting NO “scavenging” in platelets.
Conclusions
It therefore appears that acute hyperglycaemia markedly impairs vascular endothelial function, primarily via diminished NO formation, and also impairs NO signalling, mainly in platelets. These findings constitute a compelling argument for limiting hyperglycaemia (for example via insulin infusion) at the time of all cardiovascular crises. The failure of the CREATE-ECLA trial (23) to improve outcomes in acute myocardial infarction should remind us that the latter was not really a study of reversal of hyperglycaemia, but rather evaluation of a strategy of increasing myocardial glucose utilization.
Acknowledgements
Cher-Rin Chong is a recipient of an NHMRC Dora Lush Biomedical Research Postgraduate Scholarship; Dr. DT Ngo is a recipient of the Hospital Research Foundation (THRF) Mid-Career Research Grant; Dr. AL Sverdlov is a recipient of the NHMRC CJ Martin Postdoctoral Fellowship (APP1037603).
Footnote
Provenance: This is a Guest Editorial commissioned by the Section Editor Yue Liu (Department of Cardiology, the First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Malmberg K, Yusuf S, Gerstein HC, et al. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 2000;102:1014-9. [PubMed]
- Pallisgaard JL, Lindhardt TB, Olesen JB, et al. Management and prognosis of atrial fibrillation in the diabetic patient. Expert Rev Cardiovasc Ther 2015;13:643-51. [PubMed]
- Kangralkar VA, Patil SD, Badivadekar RM. Oxidative stress and diabetes: A review. International Journal of Pharmaceutical Applications 2010;1:38-45.
- Siervo M, Corander M, Stranges S, et al. Post-challenge hyperglycaemia, nitric oxide production and endothelial dysfunction: the putative role of asymmetric dimethylarginine (ADMA). Nutr Metab Cardiovasc Dis 2011;21:1-10. [PubMed]
- Ishizaka M, Nagai A, Iwanaga M, et al. Possible involvement of enhanced arginase activity due to up-regulated arginases and decreased hydroxyarginine in accelerating intimal hyperplasia with hyperglycemia. Vascul Pharmacol 2007;47:272-80. [PubMed]
- Chirkov YY, Horowitz JD. Impaired tissue responsiveness to organic nitrates and nitric oxide: a new therapeutic frontier? Pharmacol Ther 2007;116:287-305. [PubMed]
- Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992;340:1111-5. [PubMed]
- Green DJ, Dawson EA, Groenewoud HM, et al. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension 2014;63:376-82. [PubMed]
- Adams MR, Robinson J, McCredie R, et al. Smooth muscle dysfunction occurs independently of impaired endothelium-dependent dilation in adults at risk of atherosclerosis. J Am Coll Cardiol 1998;32:123-7. [PubMed]
- Sorensen KE, Celermajer DS, Spiegelhalter DJ, et al. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. Br Heart J 1995;74:247-53. [PubMed]
- Loader J, Montero D, Lorenzen C, et al. Acute Hyperglycemia Impairs Vascular Function in Healthy and Cardiometabolic Diseased Subjects: Systematic Review and Meta-Analysis. Arterioscler Thromb Vasc Biol 2015;35:2060-72. [PubMed]
- Kashyap SR, Lara A, Zhang R, et al. Insulin reduces plasma arginase activity in type 2 diabetic patients. Diabetes Care 2008;31:134-9. [PubMed]
- Mah E, Noh SK, Ballard KD, et al. Postprandial hyperglycemia impairs vascular endothelial function in healthy men by inducing lipid peroxidation and increasing asymmetric dimethylarginine:arginine. J Nutr 2011;141:1961-8. [PubMed]
- Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000;355:773-8. [PubMed]
- Malmberg K, Norhammar A, Wedel H, et al. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999;99:2626-32. [PubMed]
- Worthley MI, Holmes AS, Willoughby SR, et al. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol 2007;49:304-10. [PubMed]
- Sverdlov AL, Chan WP, Procter NE, et al. Reciprocal regulation of NO signaling and TXNIP expression in humans: impact of aging and ramipril therapy. Int J Cardiol 2013;168:4624-30. [PubMed]
- Willoughby SR, Rajendran S, Chan WP, et al. Ramipril sensitizes platelets to nitric oxide: implications for therapy in high-risk patients. J Am Coll Cardiol 2012;60:887-94. [PubMed]
- Cha-Molstad H, Saxena G, Chen J, et al. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J Biol Chem 2009;284:16898-905. [PubMed]
- Chong CR, Liu S, Licari G, et al. Reversal of hyperglycemia: effects on nitric oxide signaling. Am J Med 2015;128:427-30. [PubMed]
- Chong CR, Chan WP, Nguyen TH, et al. Thioredoxin-interacting protein: pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc Drugs Ther 2014;28:347-60. [PubMed]
- Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res 2009;82:9-20. [PubMed]
- Mehta SR, Yusuf S, Díaz R, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA 2005;293:437-46. [PubMed]


