A narrative review of progress in airway stents
Introduction
Airway stents are geometric objects fixed with bio-compatible medical-grade silicone or nickel-titanium alloy. As a major component of an integrated Interventional Pulmonology service, airway stents can provide available and timely relief to patients with airway stenosis. Such stents can be metallic or silicone, based on the material. Multiple factors (i.e., indication, physician expertise, the available equipment within the endoscopy unit and specific clinical situation) should be taken into account when selecting a proper stent. Metallic stents have seen increased application in the treatment of malignant stenosis, which may be attributed to their convenience that avoids the utility of rigid bronchoscopy. Of note, uncovered metallic stents prefer the theoretical benefit of neo-epithelialization that is instrumental in normal mucociliary clearance of secretion (1,2). There was a study suggesting that even for palliative patients with malignant airway stenosis, metallic stent implantation was also a safe and effective procedure that provided rapid palliation of symptoms and improvement in patient functional status (3). However, given their significant complications and difficulties associated with removal, the United States (US) Food and Drug Administration (FDA) issued a warning against their use in patients with benign airway stenosis (4), which was validated by recent clinical evidence as well (5). In view of this, treatment in the clinical field has begun to move towards greater use of silicone stents, owing to their ease of removal and more favorable complication rate compared with metallic stents.
The clinical application of silicone stents has evolved memorably over the last few decades, beginning with the silicone T-tube designed in 1965 by Montgomery (6), followed by the Dumon stent (7), which remained safe and reliable for patients with both benign and cancer-related airway stenosis at any given moment. It should be noted that there are certain limitations with silicone stents recognized as follows: (I) insertion of silicone stents requires a rigid bronchoscopy under general anesthesia; (II) the stent-related complications, such as migration, granuloma formation, mucus plugging, infections and restenosis, must not be neglected. Hence, over the years, various novel stents have been designed to present a promising procedure of solving the limitations that “classic” silicone and metallic stents have. Table 1 shows the details of the individual stents. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1871/rc).
Table 1
| Researcher (time) | Definition | Type of stents | Manufacturer | Soft/Rigid bronchoscopy | Application |
|---|---|---|---|---|---|
| Han, et al. (2017) | Hinged and covered SEMS | Novel metallic stents | Nanjing Micro-Tech Medical Company, Nanjing, China | Soft | Feasible and safe for benign airway stenosis |
| Avoids the use of general anesthesia, bronchoscopy and cannula | |||||
| Dahlqvist, et al. (2016) | Micro-Tech fully covered SEMS | Novel metallic stents | Nanjing Co., Republic of Korea | Rigid | Easy to locate and to remove Complications are frequent when removing the stent but not life-threatening |
| Jung, et al. (2021) | A new silicone airway stent (GINA stent) with an anti-migration design, dynamic structure that enables the reduction of stent cross-sectional area, and radio-opacity | Novel silicon stents | Invented by Taehoon Lee | Rigid | Better mechanical properties and comparable short-term performance compared to the Dumon stent |
| Sigler, et al. (2015) | Stents have an anti-proliferative coating with sirolimus | Drug-eluting stents | Cypher Select, Johnson & Johnson, Cordis, USA | Soft | Not different from bare metal stents in an experimental environment |
| Unable to inhibit the formation of granulation tissue | |||||
| Fuehner, et al. (2013) | Stents are based on Polydioxanone | Biodegradable stents | Ella-Cs, Ltd., Hradec, Kralove, Czech Republic | Rigid | A useful and safe procedure for patients with airway stenosis after lung transplantation |
| Wang, et at. (2018) | Stents loaded with I125 seeds | Radioactive stents | MTN Nanjing MicroInvasive Medical (Nanjing, China) | Rigid | Less restenosis and better OS relative to bare metallic stents A safe and effective means for inoperable malignant airway stenosis |
| Hatachi, et al. (2020) | The personalized Y-shaped silicone stent using 3D printed technology | 3D printed stents | 3D printer (STRA-SYS; Eden Prairie, MN, USA); The Y-shaped silicone stent (Dumon; Novatech, La Ciotat, France) |
Rigid | Improve the symptoms of a patient with airway stenosis caused by granulation |
| Enhance the security and accuracy of stent placement |
SEMS, self-expandable metallic airway stents.
Methods
A comprehensive and systematical online literature search via PubMed, Web of Science, and EMBASE (from January 1964 to November 2021) was performed by two authors independently and the search strategy summary was shown in Table 2 as follows.
Table 2
| Items | Specification |
|---|---|
| Date of Search (specified to date, month and year) | From 15 February 2021 to 21 March 2021 |
| Databases and other sources searched | PubMed, Web of Science, and Embase |
| Search terms used (including MeSH and free text search terms and filters) | Refer to Table S1 |
| Timeframe | From 1 January 1964 to 1 November 2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Inclusion criteria: novel metallic stents; novel silicone stents; drug-eluting stents; biodegradable stents; radioactive stents; three-dimensional (3D) printed stents |
| Exclusion criteria: research with similar conclusions | |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Two investigators (S Tian, H Huang) performed the search strategy independently and then conducted a secondary retrieval of eligible studies. Then two independent researchers (S Tian, Z Hu) assessed potentially relevant articles, according to the above selection criteria, and the discrepancies were checked by performing a blind cross-check. If there were any disagreements, the inconsistencies were solved by another reviewer (Y Dong) |
| Any additional considerations, if applicable | Apart from database retrieval, the references list of eligible literature was also manually screened to identify potentially relevant researches not included in the initial search |
Novel metallic stents
Uncovered stents
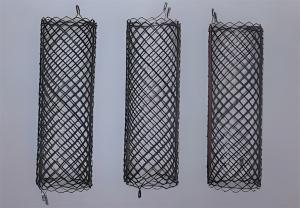
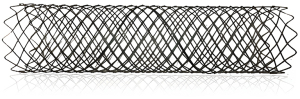
Restenosis is a frequent complication when implanting permanent stent into patients with benign tracheobronchial stenosis. To resolve the clinical dilemma, Li (8) designed a temporary uncovered airway stent (i.e., Chinese Li’s metallic stent) that provided three options of supporting force (Figure 1) adapting to different wall thicknesses. In addition, the stent was easy to remove because of sufficient flexibility to conform to tortuous airways. In clinical practice, Li’s metallic stents were successfully implanted in 4 patients with benign airway stenosis, and results showed that all patients had good symptom palliation with no severe complications after stenting. Recently, Jiang et al. (9) developed a novel through-the-scope (TTS) self-expandable metallic airway stents (SEMS) (Figure 2) delivery system that shifted an obtuse angle with over-the-wire (OTW) stent into an acute angle with TTS stent and had an outer diameter of only 2.67 mm. The aforesaid highlights made it possible for TTS SEMS to be implanted via the working channel (2.8 mm) of the flexible bronchoscope and to reduce their own shear force. It may translate to shorter placement times, greater accuracy and greater success rate when implanting stents. In their research, 36 TTS stents were placed into 25 patients with malignant central airway stenosis. 91.7% (33/36) of stents were successfully inserted and the stenosis grade of all patients improved convincingly after stent placement. It has to be mentioned that stent-related complications were common and occurred in 61.1% of patients, including mucus plugging (25%), granuloma formation (13.9%), tumor in-growth (13.9%), and hemoptysis (8.3%). Hence, larger randomized clinical trials are needed to evaluate the safety and efficacy of TTS SEMS in malignant central airway stenosis.
Covered stents
Polytetrafluoroethylene, silicone, and polyurethane, as the covering materials, endow covered stents with the additional advantages of minimizing tissue ingrowth and being deployed more easily. Currently, the structure of the tracheal bifurcation, a huge challenge for “classic” metallic stents, presents a high risk of restenosis and migration. Covered self-expanding Y-shaped stents have been introduced as a way to manage complex airway disease especially fistulization near the tracheal carina (10). Nevertheless, such stents have not been approved so far in the United States. Interestingly, in a latest retrospective analysis comparing long-term survival and complications amongst patients treated for malignant airway stenosis or tracheoesophageal fistula with Y-shaped silicon stents or covered self-expanding Y-stents, results indicated that symptom palliation, insertion safety, survival or complication rate were also found to be not different for the two types of stent (11). Fiorelli et al. (12) designed a fully-covered standard conical SEMS (CSEMS) as an emergency treatment for a patient with complex airway malignant stenosis, and the dyspnea immediately improved significantly after stent placement. This may be attributed to excellent conformation to the anatomy of complex and tortuous airways. In addition, it was also reported that multiple covered, bifurcated SEMS showcased high degrees of safety and efficacy for complex tracheobronchial stenosis or fistulas (13).
Apart from resulting in migration for complex airway stenosis, “classic” metallic stents can also cause certain immune rejections and poor matchings. In view of this, Wu et al. (14) developed an airway SEMS based on nano-technology surface modification. A total of 42 patients with airway stenosis were randomly divided into the experimental group (SEMS based on nano-technology surface modification) and the control group (Ni-Ti memory alloy stents), with 21 patients in each group. Results revealed that in contrast to the control group, the lumen diameter of the airway stenosis, forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) levels in the experimental group was higher, and the incidence rate of complications significantly lower (9.52% vs. 19.05%, P<0.05). Furthermore, a novel fully-covered SEMS (AERO) was designed to possess the advantages of both silicone stents (i.e., ease of removal) and “classic” metallic stents (i.e., ease of placement). Ishida et al. (15) reported using the AERO stent in 36 patients with malignant airway stenosis. All patients experienced significant improvement of lung function. Migration was observed in 6 cases and no complications occurred when proceeding with stent removal.
Novel covered metallic stents are also potentially effective in the management of benign airway diseases. Menna et al. (16) reported on a large series of 74 fully-covered SEMS placed in 68 patients with inoperable tracheobronchial stenosis or postoperative bronchopleural fistulas. Improvement in symptoms was observed in all patients and stent-related complications only occurred in 20 (29.4%) patients, which demonstrated that fully-covered SEMS were effective irrespective of airway pathology. Similarly, temporary partially-covered tracheobronchial stenting (17) and a hinged SEMS (18) were proven to be effective, safe, and easy to be performed on benign airway diseases. However, other results indicated complications were frequent during stent removal (i.e., Micro-Tech FC-SEMS and third-generation SEMS) (19,20). Hence, further research is needed to validate the safety and availability of novel covered metallic stents as well as compare the performance between novel metallic stents and silicone stents for the treatment of benign airway stenosis. Given the marked structural impact of cover configuration on stent performance, it is of great clinical attention that the loading configuration that covered stents are about to be subjected to should be considered before stent placement (21).
Novel silicone stents
Multiple attempts have been performed to overcome the existing defects pertaining to “classic” silicone stents. The “Natural stent”, a newly-developed silicone airway stent with interposing flexible outer membrane, was designed to increase fixation (22). However, the Natural stent did not present any tangible benefit over the Dumon stent for managing benign tracheobronchial stenosis patients. Given the limitations in terms of shape and mechanical features of the silicone, Vearick et al. (23) tested the reinforcement of silicone using fibers, including polypropylene (PP), polyamide (PA) and carbon fiber (CF). Tensile strength and Shore A hardness testing showed that CF exhibited the best mechanical performance, and subsequent finite element compression strength tests further confirmed this result. On the basis of the aforesaid results, the stent was produced using CF and placed in the trachea of a sheep. After 1 month of stenting, the tracheal tissue presented an inflammatory course. Longer research is clearly required to test the safety and utility of the novel stent. Recently, Jung et al. (24) created a novel silicone airway stent (GINA stent) and evaluated it using a porcine model with airway stenosis. Compared with the Dumon stent, the GINA stent presented better mechanical performance, which may be attributed to design improvements made by transforming the outer ring into a right-angled triangle shape. However, human-based studies are required to confirm this hypothesis.
Drug-eluting stents
Drug-eluting stents might be a suitable alternative to “classic” stents. Besides the mechanical advantages of the stents themselves, sustained release of drugs would inhibit granuloma tissue formation and act as a local chemotherapy. Recently, Debiane et al. (25) randomly assigned 45 pigs to receive drug-eluting stents (DES) filled with either gendine (n=36) or standard silicone stents (n=9). Although there were no significant differences in granulation tissue volume, tracheal thickness, or tissue microbiology between DES and standard silicone, the DES stent surface exhibited antibacterial activity. Additionally, design-based stereology could quantify the tissue changes associated with airway stenting. Wang et al. (26) placed paclitaxel drug-eluting airway stents (an experimental group, n=4) and bare metal stents (a control group, n=4) into 8 beagles. It was found that the experimental group showed less granulation tissue formation than the control group, with a high concentration of the drug in the stented area and the adjacent area. Very low levels of drug were detected in the lung tissue, and side effects were not noted in the blood test. These findings were also validated in recent research (27). Similarly, rapamycin-eluting stents were studied in a mouse model of laryngotracheal stenosis; results indicated that rapamycin-eluting stents had more adequate mechanical stability at 4 weeks and greater drug-release ability at 6 weeks when compared with PDLGA [Poly(DL-lactide-co-glycolide)] stents (28). Surprisingly, along with drugs, genetic material can also be transferred by airway stents (29). However, it is important to remember that in the small series created by Sigler et al. (30), drug-eluting stents and bare metal stents did not differ with respect to experimental setting. As promising as these novel stents can be, this area is still in its early stages. Recently, Hohenforst-Schmidt et al. (31) critically reviewed the value of drug-eluting stents for airways.
Biodegradable stents
The concept of biodegradable stents has gained much attention, owing to the fact that they will disintegrate gradually as time passes. It is theoretically-presumed that biodegradable stents can reduce the rate of stent-related complications (e.g., migration, granuloma formation). Recently, a survey study has showed that amongst all available airway stents in medical applications, 7.5% of them are biodegradable stents (32). There have been several studies reporting the use of these novel stents in the management of airway stenosis. Rodriguez-Zapater et al. evaluated airway reaction caused by a biodegradable polydioxanone airway stent (i.e., ELLA stent) in a rabbit model. The stent only produced a mild reaction that recovered with tracheal degeneration (33). A landmark study was done by Lischke et al. (34), who first reported the application of biodegradable stents in the clinical field. A total of 20 biodegradable stents were inserted endoscopically in six patients with post-transplant bronchial anastomotic stenosis. All patients had immediate symptomatic relief without complications after stenting. One patient suddenly died of pulmonary embolism 1 year post-implantation; the other five remained clinically-well during a 4-year follow-up period. Further research has confirmed this as well (35). Furthermore, Stehlik et al. (36) reported on the safety and efficacy of biodegradable stents in the treatment of benign airway stenosis. All of the aforesaid stents are made of bio-absorbable polydioxanone, and recent study showed that high-purity zinc and magnesium are possibly ideal materials for biodegradable stents due to satisfactory biocompatibility and appropriate corrosion (37). However, the literature mainly focused on animal trials and the sample size of related studies is small, and thus the significance of the results in clinical setting remains to be determined.
Radioactive stents
Radioactive stents have been successfully utilized in patients with malignant biliary obstruction or esophageal cancer. The deployment of radioactive stents in the management of malignant airway stenosis appears to be an attractive prospect. A prospective randomized controlled study was conducted by Wang et al. (38), who randomly assigned 66 patients with inoperable malignant airway stenosis to receive a novel bare metal stent loaded with either I125 seeds (RBMS, n=33) or a “classic” bare metal stent (CBMS, n=33). Stents were successfully implanted in all patients and the stenosis immediately improved in both groups after stent implantation. The incidence of complications after stenting was found to be equal in both groups, while the RBMS group had a longer median survival compared with the CBMS group, with statistical significance (170 vs. 123 days, P<0.05). Recently, a meta-analysis indicated that radioactive stents placement had a lower stent restenosis rate (13.7% vs. 37.8%, P<0.00001), higher 3-month survival rate (71.9% vs. 52.7%, P=0.03), and increased overall survival (OS) (P<0.0001) in comparison with normal stents placement when used to treat malignant airway stenosis, and the difference was statistically-significant (39).
Three-dimensional (3D) printed stents
The advent of 3D-printed stents can be attributed to advances in biomedical engineering. It is common knowledge that personalized management to the anatomy of complex and tortuous airways is a main clinical dilemma faced by interventional pulmonologists. 3D printing technology enables stents to be personally-tailored to patient-specific airway anatomy and all anatomical shapes, diameters, and lengths can be provided to allow rapid prototyping and onsite customization (40): first, the shape of a stent can be designed regarding the computed tomography (CT) and bronchoscopy measures; second, a computer-aided design program is employed to construct 3D model; then a construction file containing the information of this model is generated that can be transferred to the printer; in the next step, an original customized stent is produced with the stereolithography printers; following various surface treatments including grinding, polishing, dipping in solvents or liquid polymers, and sterilization, a ultimate 3D-engineered personalized airway stent is manufactured and can be applied rapidly in clinical work (41). In theory, 3D-printed stents meet all the requirements of ideal stents.
An increasing number of studies evaluated the value of 3D-printed stents in the management of airway stenosis. Guibert and colleagues (42) reported the first 3D-printed application in airway stenting for a complex post-transplant airway that could not be managed by a conventional airway stent. Immediate and significant improvements in dyspnea, quality of life and pulmonary function were observed after the operation. They then expanded the utilization of computer-aided design in other highly complex situations and obtained promising proof-of-concept outcomes that would contribute to further research on this new technique (43,44). Miyazaki et al. (45) described their experience with a 3D printed airway model in a stenosis of the intermediate bronchus after right single-lung transplantation. A Y-shaped airway stent with the fabricated orifice to ventilate the upper lobe was accurately modified on the basis of 3D-printed technology. After the easy and successful insertion, the patient's status improved. Gildea et al. (46) used a similar method (application of a patient-specific silicone airway stent designed from a 3D printed mold) to cope with complex airway stenosis caused by granulomatosis polyangiitis in two patients, bringing about a durable improvement of symptoms over 1-year follow-up. Shan et al. (47) reported a small series of 12 patients with malignant airway stenosis caused by lung cancer and esophageal cancer. All patients underwent successful implantation of personalized 3D-printed stents, and dyspnea was significantly and immediately relieved in 11 patients after stent placement. There was significant improvement in the Hugh-Jones and Karnofsky performance status classification of patients after stenting relative to those before stenting, with statistical significance. Morrison et al. (48) placed patient-specific airway stents using 3D-printed technology into three infants with severe tracheobronchomalacia. Life-threatening airway diseases did not occur in all infants and two out of the three infants could be completely free from mechanical ventilation. Hatachi et al. (49) performed successful placement of a patient-specific Y-shaped silicone stent created from a 3D-printed airway model in a patient with airway stenosis caused by granulation. The patient’s symptoms improved after stenting and post-operative bronchoscopy and CT scan indicated that all main airways were open. Similarly, Cheng et al. (50) developed and implanted a personalized 3D-printed Montgomery T-tube into a patient with a tracheal anastomotic dehiscence. Follow-up bronchoscopy and CT imaging revealed no granulation tissue at 4 weeks. The patient was capable of phonating when discharging himself from hospital.
Other relatively recent studies showed that personalized 3D-printed stents were a safe and effective alternative to classic stents in the treatment of patients with tracheobronchomalacia (51) and inoperable malignant airway stenosis (52). Additionally, a 3D-printed personalized airway stent that integrated the above-mentioned new-designed GINA stent with a flexible structure and an easy positioning was also developed by the research group of Kim. As a proof of concept, they implanted this 3D-engineered personalized GINA stent into two pigs of which the tracheal stenosis was at least 50%. Three weeks after stenting, the stent remained in situ, and at both ends of which, neither overgrowth of granulation tissue nor mucus plugging was seen (53).
As prospective as this technique may be, 3D-printed stents 3% were seldom deployed all over the world (32), which might be interpreted by the high costs involved and the limited clinical experience. Thus, a randomized study with a larger patient cohort and a combination of 3D-printed stents and conventional devices is needed to better confirm aforementioned findings.
Conclusions
Progress in airway stents over the years have mainly been in offering more choice for patients with airway stenosis, especially complex and benign airway lesions. However, very few of the novel stents entered routine medical environment and ideal stents have, regretfully, not yet been designed. For the time being, among commercially available stents, Ultraflex (covered SEMS) and Dumon (silicone stent) remain the commonest type of stent used all over the world (32,54). 3D-printed airway stents, as the vertex of scientific and technological progress, open up a new way to resolve the therapeutic impasses. Regardless of how perfect 3D-printed stent is, it is still a foreign body and side effects seem inevitable. Therefore, a combination of 3D-printing method and biodegradable material may present a promising avenue for future treatments of airway stenosis. It is important to note that stent implantation is only a component of an integrated treatment, and the multidisciplinary management strategy using both airway stents and the standard treatment of primary disease should be the preferred procedure.
Acknowledgments
Funding: This work was supported by 234 Climbing the Discipline Program of the First Affiliated Hospital of Naval Medical University (2020YXK026), and the Interdisciplinary Innovation Center Program of Medicine and Engineering of University of Shanghai for Science and Technology (1021302410).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1871/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1871/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1871/coif). All authors report that the study was funded by 234 Climbing the Discipline Program of the First Affiliated Hospital of Naval Medical University (2020YXK026) and the Interdisciplinary Innovation Center Program of Medicine and Engineering of University of Shanghai for Science and Technology (1021302410). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jantz MA, Silvestri GA. Silicone Stents versus Metal Stents for Management of Benign Tracheobronchial Disease Pro: Metal Stents. J Bronchology Interv Pulmonol 2000;7:177-83.
- Nashef SA, Dromer C, Velly JF, et al. Expanding wire stents in benign tracheobronchial disease: indications and complications. Ann Thorac Surg 1992;54:937-40. [Crossref] [PubMed]
- Tjahjono R, Chin RY, Flynn P. Tracheobronchial stents in palliative care: a case series and literature review. BMJ Support Palliat Care 2018;8:335-9. [Crossref] [PubMed]
- FDA public health notification: Complications from metallic tracheal stents in patients with benign airway disorders. FDA. 2005. Available online: www.fda.gov/cdrh/safety/072905-tracheal.html, accessed November 26, 2017.
- Jeong BH, Ng J, Jeong SH, et al. Outcomes of Complications Following Self-Expandable Metallic Stent Insertion for Benign Tracheobronchial Stenosis. Medicina (Kaunas) 2020;56:367. [Crossref] [PubMed]
- MONTGOMERY WW. T-TUBE TRACHEAL STENT. Arch Otolaryngol 1965;82:320-1. [Crossref] [PubMed]
- Dumon JF. A dedicated tracheobronchial stent. Chest 1990;97:328-32. [Crossref] [PubMed]
- Li Q. Temporary Metallic Stents Placement for the Treatment of Benign Airway Stenosis. Compilation of papers of the fifth national thoracic tumor and endoscopy academic conference of Chinese Medical Association. 2011:13. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CPFD&dbname=CPFD0914&filename=ZHYX201103002015&uniplatform=NZKPT&v=J9047qkLPE-zJ6NID_QbCrPMCIoVqUF0JB03mytPIf1Lc5-7qAb9-SF101j8kHx07X7a5SMgn_o%3d.
- Jiang JH, Zeng DX, Wang CG, et al. A Pilot Study of a Novel through-the-Scope Self-Expandable Metallic Airway Stents Delivery System in Malignant Central Airway Obstruction. Can Respir J 2019;2019:7828526. [Crossref] [PubMed]
- Madan K, Dhooria S, Sehgal IS, et al. A Multicenter Experience With the Placement of Self-Expanding Metallic Tracheobronchial Y Stents. J Bronchology Interv Pulmonol 2016;23:29-38. [Crossref] [PubMed]
- Sökücü SN, Özdemir C, Tural Önür S, et al. Comparison of silicon and metallic bifurcated stents in patients with malignant airway lesions. Clin Respir J 2020;14:198-204. [Crossref] [PubMed]
- Fiorelli A, Caterino U, Raucci A, et al. A conical self-expanding metallic stent for the management of critical complex tracheobronchial malignant stenosis. Interact Cardiovasc Thorac Surg 2017;24:293-5. [PubMed]
- Bi Y, Li J, Yu Z, et al. Multiple Bifurcated Covered Self-Expanding Metallic Stents for Complex Tracheobronchial Fistulas or Stenosis. Cardiovasc Intervent Radiol 2019;42:426-32. [Crossref] [PubMed]
- Wu FJ, Yao YW, Chen EG, et al. Clinical study on the treatment of tracheal stenosis with airway self-expanding metal stent based on nano-technology surface modification. Chin Mod Doctor 2017;55:14-8.
- Ishida A, Oki M, Saka H. Fully covered self-expandable metallic stents for malignant airway disorders. Respir Investig 2019;57:49-53. [Crossref] [PubMed]
- Menna C, Poggi C, Ibrahim M, et al. Coated expandable metal stents are effective irrespective of airway pathology. J Thorac Dis 2017;9:4574-83. [Crossref] [PubMed]
- Ma J, Han X, Wu G, et al. Outcomes of Temporary Partially Covered Stent Placement for Benign Tracheobronchial Stenosis. Cardiovasc Intervent Radiol 2016;39:1144-51. [Crossref] [PubMed]
- Han X, Al-Tariq Q, Zhao Y, et al. Customized Hinged Covered Metallic Stents for the Treatment of Benign Main Bronchial Stenosis. Ann Thorac Surg 2017;104:420-5. [Crossref] [PubMed]
- Dahlqvist C, Ocak S, Gourdin M, et al. Fully Covered Metallic Stents for the Treatment of Benign Airway Stenosis. Can Respir J 2016;2016:8085216. [Crossref] [PubMed]
- Fortin M, Lacasse Y, Elharrar X, et al. Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis. Respiration 2017;93:430-5. [Crossref] [PubMed]
- McGrath DJ, O'Brien B, Bruzzi M, et al. Evaluation of cover effects on bare stent mechanical response. J Mech Behav Biomed Mater 2016;61:567-80. [Crossref] [PubMed]
- Ryu YJ, Kim H, Yu CM, et al. Comparison of natural and Dumon airway stents for the management of benign tracheobronchial stenoses. Respirology 2006;11:748-54. [Crossref] [PubMed]
- Vearick SB, Demétrio KB, Xavier RG, et al. Fiber-reinforced silicone for tracheobronchial stents: An experimental study. J Mech Behav Biomed Mater 2018;77:494-500. [Crossref] [PubMed]
- Jung HS, Chae G, Kim JH, et al. The mechanical characteristics and performance evaluation of a newly developed silicone airway stent (GINA stent). Sci Rep 2021;11:7958. [Crossref] [PubMed]
- Debiane L, Reitzel R, Rosenblatt J, et al. A Design-Based Stereologic Method to Quantify the Tissue Changes Associated with a Novel Drug-Eluting Tracheobronchial Stent. Respiration 2019;98:60-9. [Crossref] [PubMed]
- Wang T, Zhang J, Wang J, et al. Paclitaxel Drug-eluting Tracheal Stent Could Reduce Granulation Tissue Formation in a Canine Model. Chin Med J (Engl) 2016;129:2708-13. [Crossref] [PubMed]
- Xu J, Ong HX, Traini D, et al. Paclitaxel-eluting silicone airway stent for preventing granulation tissue growth and lung cancer relapse in central airway pathologies. Expert Opin Drug Deliv 2020;17:1631-45. [Crossref] [PubMed]
- Duvvuri M, Motz K, Murphy M, et al. Engineering an immunomodulatory drug-eluting stent to treat laryngotracheal stenosis. Biomater Sci 2019;7:1863-74. [Crossref] [PubMed]
- Kruklitis RJ, Fishbein I, Singhal S, et al. Stent-mediated gene delivery for site-specific transgene administration to the airway epithelium and management of tracheobronchial tumors. Respiration 2014;88:406-17. [Crossref] [PubMed]
- Sigler M, Klötzer J, Quentin T, et al. Stent implantation into the tracheo-bronchial system in rabbits: histopathologic sequelae in bare metal vs. drug-eluting stents. Mol Cell Pediatr 2015;2:10. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Pitsiou G, et al. Drug Eluting Stents for Malignant Airway Obstruction: A Critical Review of the Literature. J Cancer 2016;7:377-90. [Crossref] [PubMed]
- Mathew R, Hibare K, Dalar L, et al. Tracheobronchial stent sizing and deployment practices airway stenting practices around the world: a survey study. J Thorac Dis 2020;12:5495-504. [Crossref] [PubMed]
- Rodriguez-Zapater S, Serrano-Casorran C, Guirola JA, et al. Reactivity Study of a Biodegradable Polydioxanone Tracheal Stent in a Rabbit Model. Arch Bronconeumol 2020;56:643-50. (Engl Ed). [Crossref] [PubMed]
- Lischke R, Pozniak J, Vondrys D, et al. Novel biodegradable stents in the treatment of bronchial stenosis after lung transplantation. Eur J Cardiothorac Surg 2011;40:619-24. [Crossref] [PubMed]
- Fuehner T, Suhling H, Greer M, et al. Biodegradable stents after lung transplantation. Transpl Int 2013;26:e58-60. [Crossref] [PubMed]
- Stehlik L, Hytych V, Letackova J, et al. Biodegradable polydioxanone stents in the treatment of adult patients with tracheal narrowing. BMC Pulm Med 2015;15:164. [Crossref] [PubMed]
- Li Y, Yan J, Zhou W, et al. In vitro degradation and biocompatibility evaluation of typical biodegradable metals (Mg/Zn/Fe) for the application of tracheobronchial stenosis. Bioact Mater 2019;4:114-9. [Crossref] [PubMed]
- Wang Y, Lu J, Guo JH, et al. A Novel Tracheobronchial Stent Loaded with 125I Seeds in Patients with Malignant Airway Obstruction Compared to a Conventional Stent: A Prospective Randomized Controlled Study. EBioMedicine 2018;33:269-75. [Crossref] [PubMed]
- Meng QK, Yu XY, Li W, et al. Radioactive and normal stent insertion for the treatment of malignant airway stenosis: A meta-analysis. Brachytherapy 2021;20:883-91. [Crossref] [PubMed]
- Guibert N, Mhanna L, Didier A, et al. Integration of 3D printing and additive manufacturing in the interventional pulmonologist's toolbox. Respir Med 2018;134:139-42. [Crossref] [PubMed]
- Freitag L, Gördes M, Zarogoulidis P, et al. Towards Individualized Tracheobronchial Stents: Technical, Practical and Legal Considerations. Respiration 2017;94:442-56. [Crossref] [PubMed]
- Guibert N, Didier A, Moreno B, et al. Treatment of Post-transplant Complex Airway Stenosis with a Three-Dimensional, Computer-assisted Customized Airway Stent. Am J Respir Crit Care Med 2017;195:e31-3. [Crossref] [PubMed]
- Guibert N, Didier A, Moreno B, et al. Treatment of complex airway stenoses using patient-specific 3D-engineered stents: a proof-of-concept study. Thorax 2019;74:810-3. [Crossref] [PubMed]
- Guibert N, Moreno B, Hermant C. Usefulness of 3D Printing to Manage Complex Tracheal Stenosis. J Bronchology Interv Pulmonol 2017;24:e27-9. [Crossref] [PubMed]
- Miyazaki T, Yamasaki N, Tsuchiya T, et al. Airway stent insertion simulated with a three-dimensional printed airway model. Ann Thorac Surg 2015;99:e21-3. [Crossref] [PubMed]
- Gildea TR, Young BP, Machuzak MS. Application of 3D Printing for Patient-Specific Silicone Stents: 1-Year Follow-Up on 2 Patients. Respiration 2018;96:488-94. [Crossref] [PubMed]
- Shan Q, Huang W, Shang M, et al. Customization of stent design for treating malignant airway stenosis with the aid of three-dimensional printing. Quant Imaging Med Surg 2021;11:1437-46. [Crossref] [PubMed]
- Morrison RJ, Hollister SJ, Niedner MF, et al. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci Transl Med 2015;7:285ra64. [Crossref] [PubMed]
- Hatachi G, Matsumoto K, Miyazaki T, et al. Enhanced airway stenting using a preoperative, three-dimensionally printed airway model simulation. Gen Thorac Cardiovasc Surg 2020;68:1591-3. [Crossref] [PubMed]
- Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest 2015;148:e106-8. [Crossref] [PubMed]
- Bellia-Munzon G, Cieri P, Toselli L, et al. Resorbable airway splint, stents, and 3D reconstruction and printing of the airway in tracheobronchomalacia. Semin Pediatr Surg 2021;30:151063. [Crossref] [PubMed]
- Shan Q, Huang W, Wang Z, et al. Preliminary Experience With a Novel Metallic Segmented Transcordal Stent Modified With Three-Dimensional Printing for Inoperable Malignant Laryngotracheal Stenosis. Front Oncol 2021;11:619781. [Crossref] [PubMed]
- Kim JH, Lim S, Kim DH, et al. 3D-engineered personalized airway stent (custom GINA stent): Introduction and performance evaluation in pigs. Respir Med Res 2021; [Epub ahead of print]. [PubMed]
- Dutau H, Breen D, Bugalho A, et al. Current Practice of Airway Stenting in the Adult Population in Europe: A Survey of the European Association of Bronchology and Interventional Pulmonology (EABIP). Respiration 2018;95:44-54. [Crossref] [PubMed]