
Cluster phenotypes in a non-idiopathic pulmonary fibrosis fibrotic interstitial lung diseases cohort in Singapore
Introduction
Interstitial lung diseases (ILD) may demonstrate fibrosis and progressive deterioration, of which, idiopathic pulmonary fibrosis (IPF) is the most aggressive (1,2). Other ILD types may demonstrate similar behaviour, although disease patterns are more heterogenous (3-8). These are termed, “progressive fibrosing interstitial lung disease (PF-ILD)”, however a consensus definition has yet to be established and variable trajectories within each diagnosis may limit clinical utility.
Cluster analysis is a statistical method of classifying individuals into groups based on characteristic differences (9). It has been used in Western populations to describe ILD phenotypes (10-12). Asia is a culturally and ethnically heterogenous population but there is lack of Asian data on ILD, particularly in South-East Asia (13-16). This study aims to use cluster analysis to describe clinical phenotypes in a South-East Asian population of non-IPF fibrotic ILD (F-ILD) patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-40/rc).
Methods
Study subjects
Patients with ILD were recruited at diagnosis between 5 April 2012 to 4 April 2020 from the outpatient ILD clinic at Singapore General Hospital, a university-affiliated tertiary referral hospital (Appendix 1). Patients were followed up until death, lung transplantation, or censor date 30 June 2021. ILD diagnosis was based on prevailing ATS/ERS guidelines at time of diagnosis (2,17-19). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Singhealth centralised institutional review board (CIRB Reference No. 2018/2474; Protocol No. 2012/245/C). Written informed consent was obtained from all participants.
Study design and methods
At recruitment, patients’ baseline clinical data were assessed (Appendix 1). Each patient’s Gender-Age-Physiology (GAP) score was calculated and assigned their respective GAP stage (20). The GAP Index was chosen over the ILD-GAP Index as some patients had ILD diagnoses which did not conform to the ILD categories in the ILD-GAP index (20,21). Primary outcome was all-cause mortality with lung transplantation as competing risk. Secondary outcomes were respiratory-related mortality and longitudinal lung function.
Statistical analysis
Both numerical and categorical data were selected for cluster analysis based on clinical relevance and previous literature (Supplementary Material Appendix 1 Methods). Subjects who were unable to perform diffusion capacity of the lung for carbon monoxide (DLCO) (n=72) were assigned a value of 0 for unsupervised hierarchical clustering and handled as missing data for other analysis of diffusion capacity. All other data fields used in cluster analysis were complete for all subjects. Statistical analysis was performed using R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Non-metric multidimensional scaling (NMDS) and hierarchical cluster analysis was performed using “cluster” R package. A Gower dissimilarity matrix was calculated using “daisy” function and “sammon” function was applied on this matrix using a “k”-value of 6, which was determined by a scree plot (Figure S1). All subjects were embedded into a Euclidean space of k =6. Ward’s minimum-variance unsupervised hierarchical clustering was applied on this transformed dataset using an agglomerative approach with ‘hclust’ function. “Nbclust” R package was used to determine the optimal number of derivation clusters, which was 4 (Figure S2).
Differences between groups were analyzed using chi-square tests or Fischer’s exact tests for categorical data, Kruskal-Wallis tests for nonparametric continuous data and ANOVA for parametric continuous data. Survival analysis was performed using “survival” and “survminer” R-packages. All-cause mortality was assessed and compared using Kaplan-Meier curves, log-rank test and Cox proportional hazards regression model by cluster. Respiratory-related mortality was compared with non-respiratory causes as competing risk using “cmprsk” R-package.
Linear mixed effects modelling using “lme4” R-package, was used to describe the temporal relationship between clusters and lung function (10,22). The model was built using “lmer” function, using time and cluster as fixed effects and the random effects modelled uncorrelated random intercepts and slopes for the effect of time on each subject. Time points were set at 0, 6, 12, 18, 24, 36, 48, 60, 72, 84, 96 and 108 months. Forced vital capacity (FVC) measurements were attributed to those time points using a ±6-month window, using the nearest measurement to the specific time point for analysis (3). Lung function trends over time between clusters was compared using “afex” R-package.
Results
Subjects
There were 305 ILD patients recruited, of which 287 had fibrotic radiological changes and complete baseline data (Figure 1). Of the 287 subjects, 43 (15.0%) had histopathological diagnosis from surgical lung biopsy or cryobiopsy and 90 (31.4%) had bronchoalveolar lavage (BAL) cell counts. There were 86 subjects with IPF and 201 with F-ILD. Amongst non-IPF diagnosis, the most common was connective tissue disease-related ILD (CTD-ILD) (n=107, 53.2%), followed by idiopathic non-specific interstitial pneumonia (NSIP) (n=57, 28.4%), chronic hypersensitivity pneumonitis (HP) (n=19, 9.45%), unclassifiable ILD (n=8, 3.98%), desquamative interstitial pneumonia (DIP) (n=5, 2.49%) and occupational-related ILD (n=5, 2.49%) (Figure 1). The characteristics of IPF and F-ILD subjects are summarized in Table 1.
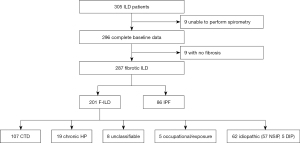
Table 1
| Variable | IPF (n=86) | Non-IPF (n=201) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 70.3±8.75 | 65.0±12.3 | <0.001 |
| Male, n (%) | 80 (93.0) | 123 (61.2) | <0.001 |
| Ethnicity, n (%) | 0.491 | ||
| Chinese | 63 (73.3) | 129 (64.2) | 0.174 |
| Malay | 7 (8.14) | 21 (10.4) | 0.699 |
| Indian | 15 (17.4) | 46 (22.9) | 0.382 |
| Others | 1 (1.16) | 5 (2.59) | 0.789 |
| Smoker/ex-smoker, n (%) | 57 (66.3) | 55 (27.4) | <0.001 |
| No. of pack years (IQR) | 40.0 (20.0, 50.0) | 25.0 (10.0, 40.0) | <0.001 |
| Weight loss at presentation, n (%) | 29 (33.7) | 79 (39.3) | 0.447 |
| BMI (kg/m2), mean ± SD | 24.1±4.29 | 25.8±7.29 | 0.053 |
| Family history of ILD, n (%) | 2 (2.33) | 5 (2.49) | 1.000 |
| Comorbid burden, n (%) | |||
| Low (0–1) | 26 (30.2) | 75 (37.3) | 0.310 |
| Moderate (2–3) | 39 (45.3) | 88 (43.8) | 0.908 |
| High (≥4) | 21 (24.4) | 38 (18.9) | 0.369 |
| Diabetes mellitus, n (%) | 35 (40.7) | 50 (24.9) | 0.011 |
| Hypertension, n (%) | 46 (53.5) | 97 (48.3) | 0.495 |
| Hyperlipidemia, n (%) | 58 (67.4) | 99 (49.3) | 0.007 |
| Ischemic heart disease, n (%) | 30 (34.9) | 32 (15.9) | <0.001 |
| Thyroid disease, n (%) | 4 (4.65) | 23 (11.4) | 0.113 |
| GERD, gastritis, peptic ulcer disease, n (%) | 9 (10.5) | 24 (11.9) | 0.875 |
| Cancer, n (%) | 5 (5.81) | 21 (10.4) | 0.304 |
| Previous history of pulmonary tuberculosis, n (%) | 9 (10.5) | 7 (3.48) | 0.037 |
| Genetic syndrome, n (%) | 2(2.33) | 0 (0.0) | 0.163 |
| Pulmonary hypertension on 2D Echo, n (%) | 29 (33.7) | 74 (36.8) | 0.714 |
| Radiology, n (%) | |||
| Emphysema | 15 (17.4) | 11 (5.47) | 0.003 |
| UIP pattern | 83 (96.5) | 15 (7.46) | <0.001 |
| FVC mean value (L), mean ± SD | 2.25±0.59 | 1.73±0.63 | <0.001 |
| FVC percentage of predicted value (%), mean ± SD | 69.1±17.8 | 62.1±17.4 | 0.002 |
| DLCO* mean value (mmol/min/kPa), mean ± SD | 4.36±2.16 | 4.78±2.87 | 0.278 |
| DLCO* percentage of predicted value (%), mean ± SD | 52.3±21.3 | 56.8±17.1 | 0.093 |
| TLC# mean value (L), mean ± SD | 3.84±0.68 | 3.28±0.86 | <0.001 |
| TLC# percentage of predicted value (%), mean ± SD | 73.0±13.6 | 75.6±17.7 | 0.293 |
| Immunosuppression, n (%) | 8 (9.30) | 154 (76.6) | <0.001 |
| Antifibrotics, n (%) | 8 (9.30) | 10 (4.98) | 0.016 |
*, 14 (16.3%) IPF patients and 58 (28.9%) non-IPF fibrotic ILD patients were unable to perform the test; #, 23 (26.7%) IPF patients and 47 (23.4%) non-IPF fibrotic ILD patients were unable to perform the test. IPF, idiopathic pulmonary fibrosis; ILD, interstitial lung disease; IQR, interquartile range; BMI, body mass index; GERD, gastroesophageal reflux disease; UIP, usual interstitial pneumonia; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; TLC, total lung capacity.
Baseline characteristics of clusters
Unsupervised hierarchical clustering of F-ILD subjects identified four clusters (Figure 2). The phenotypes are summarized in Table 2. All four clusters had subjects diagnosed with different types of ILD and radiological patterns (Table S1). Cluster 1 subjects (n=53, 26.4%) were the oldest and predominantly Chinese. They had higher body mass index (BMI), greater proportion of subjects with ischemic heart disease (IHD) and high comorbidity burden. Subjects had higher baseline FVC percentage predicted and DLCO. Those who underwent BAL had a higher proportion of neutrophilic cell counts (Table S2).
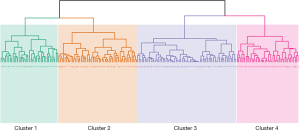
Table 2
| Variable | Cluster 1 (n=53) | Cluster 2 (n=67) | Cluster 3 (n=42) | Cluster 4 (n=39) | P value |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 71.5±7.91 | 58.1±13.1 | 63.7±11.2 | 69.6±10.5 | <0.001 |
| Male, n (%) | 18 (34.0) | 2 (2.99) | 38 (90.5) | 20 (51.3) | <0.001 |
| Ethnicity, n (%) | |||||
| Chinese | 51 (96.2) | 45 (67.2) | 33 (78.6) | 0 (0.0) | <0.001 |
| Malay | 0 (0.0) | 10 (14.9) | 5 (11.9) | 6 (15.4) | 0.047 |
| Indian | 0 (0.0) | 12 (17.9) | 4 (9.52) | 30 (76.9) | <0.001 |
| Others | 2 (3.77) | 0 (0.0) | 0 (0.0) | 3 (7.69) | 0.052 |
| Smoker/ex-smoker, n (%) | 14 (26.4) | 3 (4.48) | 30 (71.4) | 8 (20.5) | <0.001 |
| No. of pack years (IQR) | 20 (7.5, 55) | 10 (10, 15) | 32.5 (13.5, 40) | 20 (12.5, 60) | <0.001 |
| BMI (kg/m2), mean ± SD | 25.5±7.14 | 24.7±5.33 | 25.0±3.75 | 28.7±11.6 | 0.008 |
| Family history of ILD, n (%) | 0 (0.0) | 2 (2.99) | 1 (2.38) | 2 (5.13) | 0.627 |
| Comorbid burden, n (%) | |||||
| Low (0–1) | 3 (5.66) | 39 (58.2) | 26 (61.9) | 7 (17.9) | <0.001 |
| Moderate (2–3) | 33 (62.3) | 23 (34.3) | 11 (26.1) | 21 (53.8) | 0.002 |
| High (4–6) | 17 (32.1) | 5 (7.46) | 5 (11.9) | 11 (28.2) | 0.004 |
| Diabetes mellitus, n (%) | 23 (43.4) | 6 (8.96) | 5 (11.9) | 16 (41.0) | <0.001 |
| Hypertension, n (%) | 42 (79.2) | 12 (17.9) | 14 (33.3) | 29 (74.4) | <0.001 |
| Hyperlipidemia, n (%) | 44 (83.0) | 17 (25.4) | 12 (28.6) | 26 (66.7) | <0.001 |
| Ischemic heart disease, n (%) | 14 (26.4) | 1 (1.49) | 5 (11.9) | 12 (30.8) | <0.001 |
| Thyroid disease, n (%) | 5 (9.43) | 11 (16.4) | 0 (0.0) | 7 (17.9) | 0.008 |
| Asthma, n (%) | 0 (0.0) | 2 (2.99) | 0 (0.0) | 3 (7.69) | 0.070 |
| Cancer, n (%) | 5 (9.43) | 5 (7.46) | 7 (16.7) | 4 (10.3) | 0.363 |
| GERD, gastritis, peptic ulcer disease, n (%) | 11 (20.8) | 5 (7.46) | 5 (11.9) | 3 (7.69) | 0.186 |
| Previous history of pulmonary tuberculosis, n (%) | 0 (0.0) | 1 (1.49) | 1 (2.38) | 5 (12.8) | 0.021 |
| Pulmonary hypertension on 2D echo, n (%) | 19 (35.8) | 23 (34.3) | 17 (40.5) | 15 (38.5) | 0.946 |
| Groundglass, n (%) | 42 (79.2) | 57 (85.1) | 31 (73.8) | 33 (84.6) | <0.001 |
| Emphysema, n (%) | 1 (1.89) | 0 (0.0) | 9 (21.4) | 1 (2.56) | <0.001 |
| UIP pattern, n (%) | 4 (7.55) | 4 (5.97) | 4 (9.52) | 4 (10.3) | 1.000 |
| Positive ANA ≥1:160 | 12 (22.6) | 49 (73.1) | 15 (35.7) | 10 (25.6) | <0.001 |
| FVC percentage of predicted value (%), mean ± SD | 65.7±16.7 | 61.0±16.6 | 69.0±16.3 | 51.6±16.4 | <0.001 |
| DLCO percentage of predicted value (%)*, mean ± SD | 54.3±18.6 | 59.2±15.6 | 62.4±16.1 | 46.2±14.5 | <0.001 |
*, 15 patients from Cluster 1, 25 patients from Cluster 2, 1 patient from Cluster 3 and 17 patients from Cluster 4 were unable to perform the test. IQR, interquartile range; BMI, body mass index; ILD, interstitial lung disease; GERD, gastroesophageal reflux disease; UIP, usual interstitial pneumonia; ANA, antinuclear antibody; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide.
Cluster 2 subjects (n=67, 33.3%) were the youngest and mainly non-smoking females. The predominant diagnosis was CTD-ILD (Table S1). They had low comorbidity burden but higher proportion of thyroid disease. A greater proportion had groundglass changes on chest high-resolution computed tomography scan (HRCT) and antinuclear antibody (ANA) titre ≥1:160. Subjects had lower baseline FVC percentage predicted and higher DLCO percentage predicted. They had the highest proportion of immunosuppression use (Table 3).
Table 3
| Treatment, n (%) | Cluster 1 (n=53) | Cluster 2 (n=67) | Cluster 3 (n=42) | Cluster 4 (n=39) | P value |
|---|---|---|---|---|---|
| Immunosuppression | 43 (81.1) | 57 (85.1) | 22 (52.4) | 32 (82.1) | <0.001 |
| Prednisolone | 43 (81.1) | 54 (80.6) | 27 (64.3) | 33 (84.6) | 0.134 |
| Azathioprine | 9 (17.0) | 10 (14.9) | 5 (11.9) | 7 (17.9) | 0.899 |
| Mycophenolate mofetil | 14 (26.4) | 28 (41.8) | 7 (16.7) | 8 (20.5) | 0.044 |
| Cyclophosphamide | 2 (3.77) | 8 (11.9) | 2 (4.76) | 1 (2.56) | 0.218 |
| Rituximab | 2 (3.77) | 3 (4.48) | 2 (4.76) | 0 (0.0) | 0.787 |
| Antifibrotics | 0 (0.0) | 1 (1.49) | 6 (14.3) | 3 (7.69) | 0.034 |
Cluster 3 (n=42, 20.9%) comprised mainly males with heavy smoking history. They had low comorbidity burden, the highest proportion of subjects with emphysema on chest HRCT and the highest baseline lung function. They had the lowest immunosuppression use and highest antifibrotic use (Table 3); those who underwent BAL had the highest proportion of subjects with macrophagic or neutrophilic cell counts (Table S2).
Subjects in Cluster 4 (n=39, 19.4%) were mostly Indian. They had the highest BMI and highest proportion of IHD and previous pulmonary tuberculosis (TB). They had the lowest baseline lung function and highest prednisolone use (Table 3). The P values for each of the cluster’s characteristics compared against IPF are summarized in Table S3.
Survival
Kaplan-Meier curves demonstrated survival differences for all-cause mortality. Cluster 4 had the highest mortality across all time points and the shortest median survival time at 30 months (Figure 3A, Table S4) (log-rank test, P<0.001). When adjusted for treatment with immunosuppression and antifibrotics, survival differences between clusters remained significant (Figure 3B) (log-rank test, P<0.001). Cluster 4 had higher mortality risk than IPF, hazards ratio (HR) for mortality: 1.974 (HR, 1.974; 95% CI: 1.202–3.240; P=0.007). Clusters 2 and 3 had lower mortality risk than IPF, with HR for mortality: 0.105 (HR, 0.105; 95% CI: 0.037–0.295; P<0.001) and 0.413 (HR, 0.413; 95% CI: 0.198–0.858; P=0.018), respectively (Table S5).
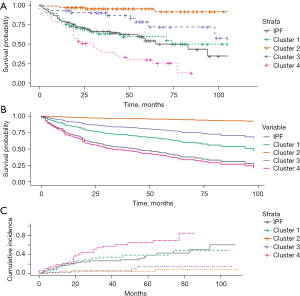
Respiratory-related mortality differences were similar to that for all-cause mortality (Figure 3C). When survival was analysed by diagnosis, CTD-ILD had lower mortality risk than IPF with HR for mortality: 0.298 (HR, 0.298; 95% CI: 0.167–0.531; P<0.001) (Table S5 and Figure S3). A higher GAP stage correlated well with mortality (Table S5 and Figure S4A) (log-rank test, P<0.001) and within each GAP stage there were significant differences in survival by cluster (Figure S4B-S4D).
Lung function trajectory
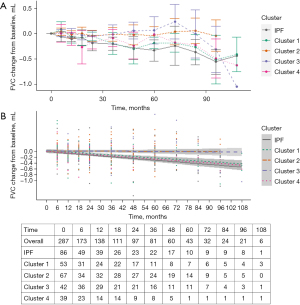
There were significant differences in lung function trajectories between clusters (Figure 4A). Cluster 4 had the lowest baseline FVC percentage predicted value and greatest FVC decline from baseline [rate of FVC decline from baseline: 55.4 (±3.88) mL/year] (Figure 4B, Table S4). This was followed by Cluster 1 [47.0 (±9.64) mL/year], whilst Cluster 3 had the slowest rate of FVC decline from baseline at 4.22 (±2.88) mL/year (Figure 4B, Table S4). Post-hoc analysis showed that there was no significant difference in the rate of FVC decline in subjects who received anti-fibrotic therapy and those who did not, for both Clusters 3 and 4 (Table S6). When analysed for a composite outcome of decline in FVC ≥5% of the predicted value from baseline or death at 12 months, there was no significant difference between clusters (Figure S5A) (log-rank test, P=0.09). There was also no significant difference between clusters when analysed for a composite outcome of decline in FVC ≥10% of the predicted value from baseline or death at 12 months (Figure S5B) (log-rank test, P=0.1).
Discussion
This is a real-world, prospective cohort study involving South-East Asian patients with F-ILD. Unsupervised hierarchal clustering classified patients into four distinct phenotypes with different outcomes and trajectories. We identified two high-risk clusters, namely Clusters 1 and 4. Cluster 1 comprised older Chinese males with high BMI, comorbidity burden, higher baseline FVC but lower DLCO, and similar mortality to IPF. Cluster 4 had the highest mortality and comprised mainly of Indians with high BMI, low baseline lung function and a higher proportion of IHD and previous pulmonary TB. Clusters 2 and 3 both had low mortality. Cluster 2 subjects were mainly younger female non-smokers with low comorbidity burden, groundglass changes on HRCT, positive ANA titre ≥1:160, lower baseline FVC but higher DLCO. Cluster 3 subjects were male smokers with low comorbidity burden, emphysema on HRCT and high baseline lung function.
Some of our findings are similar to published literature. Increasing age, male sex and poorer lung function are well-described baseline predictors of increased mortality and were demonstrated in our clusters (20,21). Previous cluster analysis also showed that younger females with positive ANA had improved survival, similar to that in Cluster 2 (10). Unclassifiable ILD and chronic HP have been identified as ILD diagnoses associated with poorer outcomes in PF-ILD cohorts (3,15,23). However, our study and other cluster analysis demonstrate that there is heterogeneity of disease behaviour within a diagnosis and overlap in disease trajectories across different diagnoses, highlighting the importance of classifying different ILDs by disease behaviour, as previously proposed in ATS/ERS guidelines (10-12,18).
Some of our findings are unique to the South-East Asian region. We found that the two high-risk clusters, Clusters 1 and 4, comprised mainly Chinese and Indian patients respectively, with Cluster 4 demonstrating a more aggressive course. In 2019, Singapore’s population distribution comprised 74.4% Chinese, 13.4% Malays, 9.0% Indians and 3.2% other ethnicities (24). In contrast, our F-ILD cohort had a lower proportion of Chinese (64.2%) and Malays (10.4%), and a higher proportion of Indians (22.9%). Differences between Asian and Caucasian ILD have been described, such as higher rates of exacerbations in East-Asians and a high proportion of chronic HP in the Indian registry (14,25). The differences in disease behaviour and prevalence by geography and ethnicity requires further research to identify potential factors such as genetic polymorphisms, environmental exposure and lifestyle practices which may account for this.
TB is endemic in Singapore and TB prevalence is high in South-East Asia (26). We found that the high-risk Cluster 4 had a high proportion of subjects with prior pulmonary TB, which is unique and of significance to TB-endemic regions. Prior pulmonary TB has been associated with poorer disease outcomes in chronic obstructive pulmonary disease (COPD) (27). Although TB incidence in ILD is 4.5 times higher than that of the general population in Israel and 15.5% of the Indian ILD registry had prior pulmonary TB, the effects of prior pulmonary TB on ILD outcomes have not been delineated (14,28). Furthermore, ethnic differences have been found to result in variations in inflammatory profiles and clinical phenotypes in TB (29,30). Given that Cluster 4 was predominantly Indian, implications of prior infection and ethnic differences on ILD outcomes require further study.
Our study also highlights the importance of identifying and managing comorbidities in ILD. We found that Clusters 1 and 4, which had high comorbidity burden and high proportion of IHD respectively, had high mortality. Increasing number of comorbidities, particularly cardiovascular risk factors and untreated cardiac disease, increases ILD mortality (11,31,32). We found that all the clusters had a mean BMI above the Asian cut-off of 23.0 kg/m2 (33). This could be related to corticosteroid therapy which 78.1% received. The effect of BMI on ILD outcomes requires further study. Low BMI and decreasing BMI trend correlates with increased mortality, however high BMI increases cardiovascular risk and is associated with increased exacerbations (34,35). Our study highlights the importance of defining the ILD comorbidome for targeted screening and early treatment of comorbidities (11,12,31,36).
Our findings demonstrate the challenges in identifying high-risk F-ILD at diagnosis (23). Current ILD risk scores emphasize lung function and diagnosis, with most developed for IPF (20,21). PF-ILD is characterised by lung function decline and increased mortality risk if untreated (3,5,8,23). Currently, there is no consensus on the PF-ILD definition and different criteria have been used in trials (5,7,8). Although we did not examine for PF-ILD based on existing trial criteria, we found that 45.8% of our F-ILD cohort were high-risk with mortality and disease progression similar to, or more aggressive than IPF. This is higher than the reported PF-ILD incidence of 13–40% (3,4,6). The higher proportion of F-ILD in our cohort with aggressive disease behaviour requires further study.
Some of the limitations are the small size and lack of validation cohort. Furthermore, only subjects with complete baseline data were included for cluster analysis, thus patients such as those unable to perform spirometry due to more advanced disease were excluded. Patients with sarcoidosis and rarer ILD types like pleuroparenchymal fibroelastosis were also not studied; thus, the applicability and generalisability to other ILD types requires further study. The extent of fibrosis radiologically was not quantified and hence identifying subjects that would benefit from antifibrotic therapy was also limited.
Obtaining supporting histopathological evidence to establish an ILD diagnosis is an important component of multidisciplinary diagnosis. However, surgical lung biopsy for ILD has an in-hospital mortality of 2%, which doubles by 90-day, and 19.1% will experience at least one surgical-related complication (37-39). Globally, only 10% of ILD patients undergo surgical lung biopsy due to advanced disease or lack of access to services (40). The utility of histopathology in establishing a multidisciplinary ILD diagnosis thus varies around the world, and is often dependent on local practices and resource availability which may restrict the applicability of ATS/ERS guidelines (13,40). Our cohort’s biopsy rate of 15.0%, is reflective of local clinical practices and patient preferences. Furthermore, as our unit is a tertiary referral centre, some patients are only referred in advanced stages and thus unable to undergo biopsy due to high risks.
Although this was a single centre study, our centre receives ILD referrals across Singapore and thus our cohort is representative of local ILD patients. Patients were diagnosed strictly according to ATS/ERS guidelines and longitudinal disease trends were characterised. However, referral bias from clinicians at referring centres may have resulted in patients assessed to be too advanced or too early in the disease to require tertiary specialist care, and hence not referred. Some patients may have received treatment prior to consultation at our institution, altering the clinical phenotype and disease trajectory; however, we found that survival differences across clusters remained significant after adjusting for treatment. In addition, due to cost limitations, such as for the use of antifibrotics, some subjects may have declined treatment and hence deteriorate more rapidly. More research is needed to identify factors which may account for differences amongst clusters.
Our study characterises the disease trajectories of South-East Asian F-ILD into four distinct clinical phenotypes. Although further validation is needed in larger multicentre cohorts, this has broader clinical utility in the management of ILD and sheds light on future areas of study with regards to disease behaviour in different South-East Asian ethnicities and comorbidities. There is currently a void in the understanding of ILD in South-East Asia and our findings are helpful in prognostication for such patients and highlight the need for further research in South-East Asia to identify high-risk groups.
Acknowledgments
Funding: This work was supported by Singapore General Hospital (grant No. SRG-NIG-04-2021 to MLW Kam).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-40/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-40/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-40/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-40/coif). MLWK has received research funding from Singapore General Hospital (SGH). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Singhealth centralised institutional review board (CIRB Reference No. 2018/2474; Protocol No. 2012/245/C). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Park JH, Kim DS, Park IN, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007;175:705-11. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Nasser M, Larrieu S, Si-Mohamed S, et al. Progressive fibrosing interstitial lung disease: a clinical cohort (the PROGRESS study). Eur Respir J 2021;57:2002718. [Crossref] [PubMed]
- Wijsenbeek M, Kreuter M, Olson A, et al. Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin 2019;35:2015-24. [Crossref] [PubMed]
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med 2019;381:1718-27. [Crossref] [PubMed]
- Brown KK, Martinez FJ, Walsh SLF, et al. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J 2020;55:2000085. [Crossref] [PubMed]
- Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2020;8:147-57. [Crossref] [PubMed]
- Behr J, Prasse A, Kreuter M, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med 2021;9:476-86. [Crossref] [PubMed]
- McLachlan GJ. Cluster analysis and related techniques in medical research. Stat Methods Med Res 1992;1:27-48. [Crossref] [PubMed]
- Adegunsoye A, Oldham JM, Chung JH, et al. Phenotypic Clusters Predict Outcomes in a Longitudinal Interstitial Lung Disease Cohort. Chest 2018;153:349-60. [Crossref] [PubMed]
- Wong AW, Lee TY, Johannson KA, et al. A cluster-based analysis evaluating the impact of comorbidities in fibrotic interstitial lung disease. Respir Res 2020;21:322. [Crossref] [PubMed]
- Prior TS, Hoyer N, Hilberg O, et al. Clusters of comorbidities in idiopathic pulmonary fibrosis. Respir Med 2021;185:106490. [Crossref] [PubMed]
- Rivera-Ortega P, Molina-Molina M. Interstitial Lung Diseases in Developing Countries. Ann Glob Health 2019;85:4. [Crossref] [PubMed]
- Singh S, Collins BF, Sharma BB, et al. Interstitial Lung Disease in India. Results of a Prospective Registry. Am J Respir Crit Care Med 2017;195:801-13. [Crossref] [PubMed]
- Kwon BS, Choe J, Chae EJ, et al. Progressive fibrosing interstitial lung disease: prevalence and clinical outcome. Respir Res 2021;22:282. [Crossref] [PubMed]
- Zhan X, Yan W, Wang Y, et al. Clinical features of anti-synthetase syndrome associated interstitial lung disease: a retrospective cohort in China. BMC Pulm Med 2021;21:57. [Crossref] [PubMed]
- American Thoracic Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91. [Crossref] [PubMed]
- Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest 2014;145:723-8. [Crossref] [PubMed]
- Edwards LJ. Modern statistical techniques for the analysis of longitudinal data in biomedical research. Pediatr Pulmonol 2000;30:330-44. [Crossref] [PubMed]
- Goos T, De Sadeleer LJ, Yserbyt J, et al. Defining and predicting progression in non-IPF interstitial lung disease. Respir Med 2021;189:106626. [Crossref] [PubMed]
- Singapore Department of Statistics/Ministry of Trade and Industry/Republic of Singapore. Population Trends, 2019. Available Online: https://www.singstat.gov.sg/-/media/files/publications/population/population2019.pdf
- Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014;190:773-9. [Crossref] [PubMed]
- Global tuberculosis report 2018. Geneva: World Health Organization, 2018.
- Park HJ, Byun MK, Kim HJ, et al. History of pulmonary tuberculosis affects the severity and clinical outcomes of COPD. Respirology 2018;23:100-6. [Crossref] [PubMed]
- Shachor Y, Schindler D, Siegal A, et al. Increased incidence of pulmonary tuberculosis in chronic interstitial lung disease. Thorax 1989;44:151-3. [Crossref] [PubMed]
- Coussens AK, Wilkinson RJ, Nikolayevskyy V, et al. Ethnic variation in inflammatory profile in tuberculosis. PLoS Pathog 2013;9:e1003468. [Crossref] [PubMed]
- Pareek M, Evans J, Innes J, et al. Ethnicity and mycobacterial lineage as determinants of tuberculosis disease phenotype. Thorax 2013;68:221-9. [Crossref] [PubMed]
- Schwarzkopf L, Witt S, Waelscher J, et al. Associations between comorbidities, their treatment and survival in patients with interstitial lung diseases - a claims data analysis. Respir Res 2018;19:73. [Crossref] [PubMed]
- Carter P, Lagan J, Fortune C, et al. Association of Cardiovascular Disease With Respiratory Disease. J Am Coll Cardiol 2019;73:2166-77. [Crossref] [PubMed]
- Expert Consultation WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157-63. [Crossref] [PubMed]
- Alakhras M, Decker PA, Nadrous HF, et al. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest 2007;131:1448-53. [Crossref] [PubMed]
- Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2010;27:103-10. [Crossref] [PubMed]
- Yagyu H, Murohashi K, Hara Y, et al. Clinical utility of a composite scoring system including Charlson Comorbidity Index score in patients with interstitial lung disease. J Thorac Dis 2020;12:5774-82. [Crossref] [PubMed]
- Hutchinson JP, McKeever TM, Fogarty AW, et al. Surgical lung biopsy for the diagnosis of interstitial lung disease in England: 1997-2008. Eur Respir J 2016;48:1453-61. [Crossref] [PubMed]
- Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg 2007;83:1140-4. [Crossref] [PubMed]
- Luo Q, Han Q, Chen X, et al. The diagnosis efficacy and safety of video-assisted thoracoscopy surgery (VATS) in undefined interstitial lung diseases: a retrospective study. J Thorac Dis 2013;5:283-8. [PubMed]
- Richeldi L, Launders N, Martinez F, et al. The characterisation of interstitial lung disease multidisciplinary team meetings: a global study. ERJ Open Res 2019;5:e00209-2018. [Crossref] [PubMed]


