Long-term respiratory function recovery in patients with stage I lung cancer receiving video-assisted thoracic surgery versus thoracotomy
Introduction
Video-assisted thoracic surgery (VATS) and thoracotomy are widely used lobectomy procedures for treatment of early non-small cell lung cancer (NSCLC). Compared with thoracotomy, VATS results in less post-operative pain, a lower inflammatory response, and shorter hospital stays (1-5). Recovery of respiration function is better with VATS because VATS does not require dissection of large thoracic muscles (6-9). However, previous studies mainly examined recovery in the first 3 months after surgery; only a few compared VATS and thoracotomy in terms of long-term recovery. Moreover, various factors influence postoperative lung function including preoperative lung function and underlying lung disease (10-13). For accuracy, the results of pulmonary function tests (PFTs) should be adjusted for these factors, but no previous studies have done this. Herein, we compared long-term pulmonary function recovery after VATS versus conventional thoracotomy by using propensity score matching.
Materials and methods
Patients
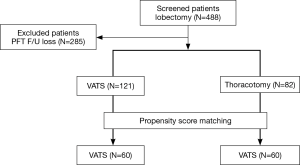
The medical records of 488 patients with pathological stage I NSCLC who underwent lobectomy between January 2005 and December 2010 at Seoul University Hospital were retrospectively reviewed. The 285 patients who were lost to follow-up and thus had no PFT data at 6 or 12 months after surgery were excluded. Among the remaining 203 patients, 121 underwent VATS and 82 underwent open thoracotomy. To compare the effects of these procedures on the recovery of pulmonary function, we used propensity score matching to create two matched groups, namely the VATS group and the thoracotomy group, each consisting of 60 patients. Propensity score matching included TNM stage, age, sex, smoking history, presence of lung disease, and preoperative pulmonary function (Figure 1). Information extracted from medical records included age, sex, comorbidities, smoking history, the results of preoperative and postoperative PFTs, and tumor stage, histology, location, and size. This study was approved by the Seoul National University Hospital Institutional Review Board (H-1204-006-403).
Operative procedures
Thoracotomy was performed by making a 20-cm posterolateral incision through the fourth or fifth intercostal space, sparing the serratus anterior muscle. VATS was performed by making three incisions with rib sparing. Two incisions were used as 10-mm thoracoscopic ports, and the third was used as a 5-mm surgical instrument port. A 5-cm access was located anteriorly in the fourth or fifth intercostal space. Complete lymph node dissection was usually carried out along with VATS or thoracotomy. The surgical approach was chosen based on clinical attributes such as tumor size and patient age, general condition, and pulmonary function. It was selected by the two attending thoracic surgeons who performed the operations.
Pulmonary function testing
Pulmonary function data, including forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and peak flow rate (PFR), were collected preoperatively and at 6 and 12 months postoperatively. For accurate prediction of pulmonary function after lobectomy, the predicted values were calculated according to the number of resected pulmonary segments as follows (5): predicted FEV1 (FVC) = preoperative FEV1 (FVC) × 19 (number of segments removed)/19. The actual postoperative recovery values of FEV1 and FVC were calculated as follows: measured postoperative value/predicted postoperative value × 100 (%). The actual ratio of postoperative functional loss to resected lung volume was calculated as follows (14): (measured postoperative value – predicted postoperative value)/predicted postoperative value × 100 (%).
Statistical analysis
Values are expressed as mean ± standard deviation or as number and percent. Data were analyzed by using the Student’s t-test for continuous variables and the chi-square for categorical variables. The Student’s t-test was used for intergroup comparisons, and the matched-pair t-test was used for intragroup comparisons. Differences with a P value <0.05 were considered significant. The SPSS 19.0 statistical program (Chicago, IL, USA) was used for the analysis.
Results
Overall baseline characteristics
A total of 203 patients were analyzed; 121 had undergone VATS, and 82 had undergone thoracotomy. After propensity score matching according to TNM stage, age, sex, smoking history, lung disease history, and preoperative pulmonary function, two groups (the VATS and thoracotomy groups) were created, each consisting of 60 patients (Figure 1). Table 1 shows the baseline characteristics of the patients in each group. There were no significant differences between the groups, excepting tumor size, which was significantly larger in the thoracotomy group. The most common histologic type in both groups was adenocarcinoma. Both groups had similar baseline PFT values.

Full table
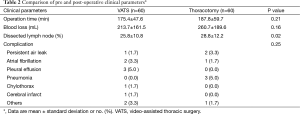
Table 2 shows the pre- and post-operative clinical parameters. Operating time and blood loss were similar in the VATS and thoracotomy groups, whereas the number of dissected lymph nodes was larger in the thoracotomy group. The lobes resected in the VATS vs. thoracotomy groups were as follows: right upper lobe, 18 (30.0%) vs. 14 (23.3%) patients; right middle lobe, 3 (5.0%) vs. 1 (1.7%) patient; right lower lobe, 15 (25.0%) vs. 15 (25.0%) patients; left upper lobe, 9 (15.0%) vs. 16 (26.7%) patients; and left lower lobe, 15 (25.0%) vs. 14 (23.3%) patients. Postoperative complications were found in 10 (16.7%) patients in the VATS group and 7 (11.7%) patients in the thoracotomy group; this difference was not significant (P=0.246).
Full table
Changes in pulmonary function with time
Postoperative reductions in FEV1 and FVC, as represented by the measured postoperative value/predicted postoperative value at 6 and 12 months after surgery, were not significantly different in the VATS and thoracotomy groups (Figure 2). The FEV1 values in these two groups were as follows: 101.60±21.14% vs. 99.30±19.20% at 6 months (P=0.535) and 101.96±21.41% vs. 100.36±19.36% at 12 months (P=0.669). The FVC values in these two groups were as follows: 98.38±21.25% vs. 93.42±18.77% at 6 months (P=0.178) and 102.00±22.48% vs. 97.57±19.71% at 12 months (P=0.253). The PFRs in these two groups were as follows: 87.62±15.80% vs. 85.13±14.04% at 6 months (P=0.362) and 88.21±16.31% vs. 86.46±15.42% at 12 months (P=0.253).

Table 3 shows the standardized functional loss ratios for FVC, FEV1, and PFR at the 6-month and 12-month follow-up. There were no significant differences in these ratios at either time between the VATS and thoracotomy groups. Figure 3 shows the functional loss ratios for FEV1 and FVC. In an intragroup analysis, the postoperative FVC in the thoracotomy group remained below the predicted postoperative value during the follow-up period and did not reach the predicted postoperative FVC (6 months/12 months: –6.58%/–2.43%). The FVC at 6 months was significantly lower than the predicted baseline FVC in this group (–6.58%, P<0.005).

Full table

Clinical outcome parameters
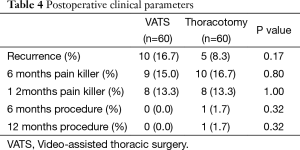
The mean follow-up duration was 55.6±20.9 months in the VATS group and 59.9±27.4 months in the thoracotomy group (P= 0.341). During the follow-up period, tumors recurred in 10 (16.7%) patients in the VATS group and 5 (8.3%) patients in the thoracotomy group (P=0.17). The analgesic requirements were similar in the VATS and thoracotomy groups: 9 (15.0%) vs. 10 (16.7%) patients at 6 months (P= 0.8) and 8 (13.3%) vs. 8 (13.3%) patients at 12 months (P=1.0), respectively. The need for procedures such as a nerve block was also similar in both groups (Table 4).
Full table
Discussion
Lobectomy with lymph node dissection is a standard treatment for stage I NSCLC, and conventional thoracotomy and VATS are popular lobectomy approaches. Thoracotomy provides excellent exposure of the thoracic organs, but requires dissection of large muscles and the ribs (15). VATS is a minimally invasive procedure that uses a small-access incision and does not require large muscle dissection. Our study compared pulmonary function and postoperative pain according to these two procedures.
Previous studies demonstrated that VATS better promoted the recovery of early postoperative pulmonary function than did conventional thoracotomy. Nakata et al. (8) showed better recovery of pulmonary function and oxygenation in patients receiving VATS compared with those receiving thoracotomy, as assessed 4, 7, and 14 days after surgery. Another study, which examined recovery up to 3 months after surgery, had a similar result (7). Because VATS causes minimal injury to the chest wall, it causes less postoperative pain and physiologic stress than thoracotomy. Our results do not dispute previous data suggesting that postoperative pain is the main impediment of short-term recovery after pulmonary resection (5,15,16).
Importantly, our results differ from those of the aforementioned studies, which evaluated early postoperative lung function. In our study, the surgical method did not significantly affect the outcomes of the PFTs in the late postoperative period. We presume that there were no differences in the rates of postoperative pain or pulmonary complications between the VATS and thoracotomy groups during this period: their need for analgesics or nerve blocks and the complication rates were similar. Therefore, we suggest that pain influences pulmonary function only in the early postoperative period and that the surgical method that generates less pain better preserves short-term pulmonary function (16). On the other hand, preservation of long-term pulmonary function depends on the volume of the resected lung, preoperative lung function, and underlying lung disease. Because it is generally believed that these factors influence postoperative pulmonary function, we adjusted for them via propensity score matching (17,18). We also calculated the predicted baseline lung function values and standardized functional loss ratios by using specific formulas adjusted for resected lung volumes.
After lobectomy, complete recovery of pulmonary function requires 6 to 12 months (19). The remaining lung tissue distends for 6 to 12 months to fill the resected part of the lung, thus promoting gas exchange (20). Serial intragroup PFT analyses showed a reduced postoperative FVC in the thoracotomy group at 6 months that did not reach the predicted baseline FVC until 12 months. In contrast, FVC in the VATS group continued to improve up to 12 months. Although there were no differences in pulmonary function recovery according to surgical method, the rate of recovery may have been lower in the thoracotomy group during the long-term follow-up period. It may be associated with the incomplete recovery of full muscle strength and further studies are needed.
The recurrence rate was higher in the VATS group than in the thoracotomy group, but not significantly so. This may reflect dissection of a lower number of lymph nodes or inadequate removal of lymph nodes in the VATS group. In support, other studies showed incomplete peribronchial and hilar lymph node evaluation in patients receiving VATS (21,22).
Our study had two limitations. First, because lung perfusion scans were not performed, postoperative lung function could not be exactly predicted. Nevertheless, this would not have appreciably affected our results because we used a formula suitable for resected lobes. Second, our study had a retrospective design and a small patient cohort. Despite these limitations, it had some strengths. We adjusted for various factors that may have affected postoperative lung function (e.g., TNM stage, age, sex, smoking history, lung disease history, and preoperative pulmonary function) via propensity score matching. For example, compensated distension of the lungs after surgery is variable in patients with lung disorders such as chronic obstructive lung disease (18). Group matching allowed us to accurately assess the impact of the surgical method on lung function. As another strength, the formula we used to calculate the postoperative predicted value was adjusted for resected lung volume. Our study provides convincing results regarding long-term lung respiratory recovery after VATS and thoracotomy that will help clinicians select the appropriate surgical method.
Conclusions
This study was a retrospective review of pulmonary function in patients who underwent VATS or thoracotomy for pathological stage I NSCLC. There were no significant differences in pulmonary function recovery during the late postoperative period after either lobectomy procedure. We suggest that the volume of the resected lung and preoperative lung function, rather than postoperative pain, are the main determinants of late recovery. However, the thoracotomy group had a worse recovery than predicted by the baseline value. Further prospective studies will be necessary owing to the limitations of this retrospective study.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [PubMed]
- Siafakas NM, Mitrouska I, Argiana E, et al. Effects of surgery on the function of the respiratory muscles. Monaldi Arch Chest Dis 1999;54:526-31. [PubMed]
- Pu Q, Ma L, Mei J, et al. Video-assisted thoracoscopic surgery versus posterolateral thoracotomy lobectomy: A more patient-friendly approach on postoperative pain, pulmonary function and shoulder function. Thorac Cancer 2013;4:84-9.
- Nakata M, Saeki H, Yokoyama N, et al. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938-41. [PubMed]
- Endoh H, Tanaka S, Yajima T, et al. Pulmonary function after pulmonary resection by posterior thoracotomy, anterior thoracotomy or video-assisted surgery. Eur J Cardiothorac Surg 2010;37:1209-14. [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [PubMed]
- Pompili C, Brunelli A, Refai M, et al. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardiothorac Surg 2010;37:525-30. [PubMed]
- Kearney DJ, Lee TH, Reilly JJ, et al. Assessment of operative risk in patients undergoing lung resection. Importance of predicted pulmonary function. Chest 1994;105:753-9. [PubMed]
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989;139:902-10. [PubMed]
- Saito H, Nakagawa T, Ito M, et al. Pulmonary function after lobectomy versus segmentectomy in patients with stage I non-small cell lung cancer. World J Surg 2014;38:2025-31. [PubMed]
- Ochroch EA, Gottschalk A, Augoustides JG, et al. Pain and physical function are similar following axillary, muscle-sparing vs posterolateral thoracotomy. Chest 2005;128:2664-70. [PubMed]
- Hazelrigg SR, Landreneau RJ, Boley TM, et al. The effect of muscle-sparing versus standard posterolateral thoracotomy on pulmonary function, muscle strength, and postoperative pain. J Thorac Cardiovasc Surg 1991;101:394-400; discussion 400-1. [PubMed]
- Kim JK, Jang SH, Lee JW, et al. Clinical parameters affecting prediction accuracy of postoperative lung function in non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2008;7:1019-23. [PubMed]
- Ladurie ML, Ranson-Bitker B. Uncertainties in the expected value for forced expiratory volume in one second after surgery. Chest 1986;90:222-8. [PubMed]
- Nagamatsu Y, Maeshiro K, Kimura NY, et al. Long-term recovery of exercise capacity and pulmonary function after lobectomy. J Thorac Cardiovasc Surg 2007;134:1273-8. [PubMed]
- Laross CD. Royal Netherlands Tuberculosis Association. Select Papers, 1979. The patient after total pneumonectomy; p. 19.
- Higuchi M, Yaginuma H, Yonechi A, et al. Long-term outcomes after video-assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg 2014;9:88. [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [PubMed]


